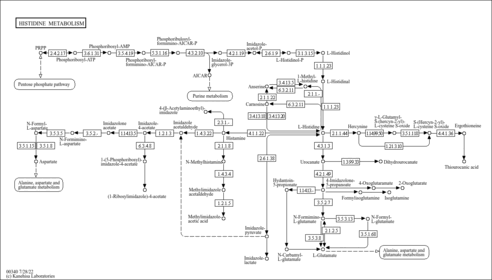
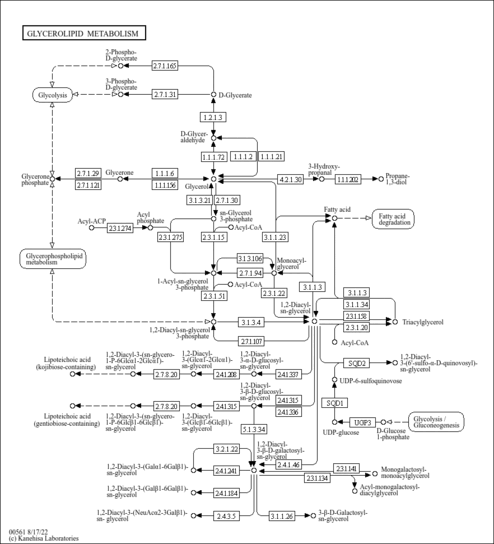
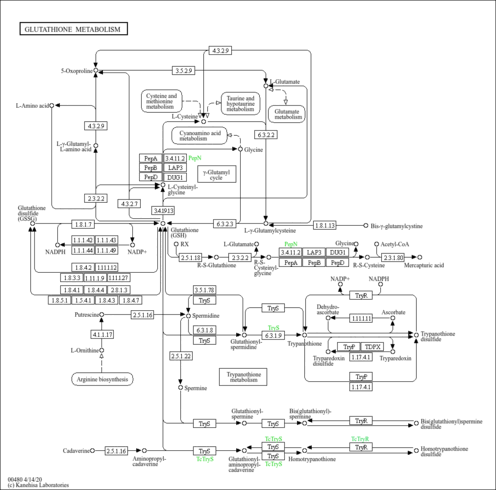
| Fatty acid synthase | FASN | 17q25 | P49327 | details |
| Methylmalonate-semialdehyde dehydrogenase [acylating], mitochondrial | ALDH6A1 | 14q24.3 | Q02252 | details |
| Aldo-keto reductase family 1 member C4 | AKR1C4 | 10p15.1 | P17516 | details |
| Carbonyl reductase [NADPH] 1 | CBR1 | 21q22.13 | P16152 | details |
| Dehydrogenase/reductase SDR family member 4 | DHRS4 | 14q11.2 | Q9BTZ2 | details |
| Carbonyl reductase [NADPH] 3 | CBR3 | 21q22.2 | O75828 | details |
| Aldose reductase | AKR1B1 | 7q35 | P15121 | details |
| Thioredoxin reductase 1, cytoplasmic | TXNRD1 | 12q23-q24.1 | Q16881 | details |
| Lathosterol oxidase | SC5DL | 11q23.3 | O75845 | details |
| Dimethylaniline monooxygenase [N-oxide-forming] 5 | FMO5 | 1q21.1 | P49326 | details |
| Heme oxygenase 2 | HMOX2 | 16p13.3 | P30519 | details |
| Squalene monooxygenase | SQLE | 8q24.1 | Q14534 | details |
| Dimethylaniline monooxygenase [N-oxide-forming] 2 | FMO2 | 1q24.3 | Q99518 | details |
| Delta-1-pyrroline-5-carboxylate dehydrogenase, mitochondrial | ALDH4A1 | 1p36 | P30038 | details |
| Thioredoxin reductase 2, mitochondrial | TXNRD2 | 22q11.21 | Q9NNW7 | details |
| Dimethylaniline monooxygenase [N-oxide-forming] 4 | FMO4 | 1q24.3 | P31512 | details |
| Dimethylaniline monooxygenase [N-oxide-forming] 3 | FMO3 | 1q24.3 | P31513 | details |
| NADPH--cytochrome P450 reductase | POR | 7q11.2 | P16435 | details |
| Dimethylaniline monooxygenase [N-oxide-forming] 1 | FMO1 | 1q24.3 | Q01740 | details |
| Methylenetetrahydrofolate reductase | MTHFR | 1p36.3 | P42898 | details |
| Glutathione reductase, mitochondrial | GSR | 8p21.1 | P00390 | details |
| Heme oxygenase 1 | HMOX1 | 22q13.1 | P09601 | details |
| NADPH:adrenodoxin oxidoreductase, mitochondrial | FDXR | 17q24-q25 | P22570 | details |
| Squalene synthase | FDFT1 | 8p23.1-p22 | P37268 | details |
| Delta(14)-sterol reductase | TM7SF2 | 11q13 | O76062 | details |
| Methylsterol monooxygenase 1 | MSMO1 | 4q32-q34 | Q15800 | details |
| Sterol-4-alpha-carboxylate 3-dehydrogenase, decarboxylating | NSDHL | Xq28 | Q15738 | details |
| Lanosterol 14-alpha demethylase | CYP51A1 | 7q21.2 | Q16850 | details |
| 4-trimethylaminobutyraldehyde dehydrogenase | ALDH9A1 | 1q23.1 | P49189 | details |
| Aldehyde dehydrogenase, dimeric NADP-preferring | ALDH3A1 | 17p11.2 | P30838 | details |
| Alpha-aminoadipic semialdehyde dehydrogenase | ALDH7A1 | 5q31 | P49419 | details |
| Aldehyde dehydrogenase family 1 member A3 | ALDH1A3 | 15q26.3 | P47895 | details |
| Aldehyde dehydrogenase, mitochondrial | ALDH2 | 12q24.2 | P05091 | details |
| Fatty aldehyde dehydrogenase | ALDH3A2 | 17p11.2 | P51648 | details |
| Aldehyde dehydrogenase X, mitochondrial | ALDH1B1 | 9p11.1 | P30837 | details |
| Pyrroline-5-carboxylate reductase 1, mitochondrial | PYCR1 | 17q25.3 | P32322 | details |
| Pyrroline-5-carboxylate reductase 2 | PYCR2 | 1q42.12 | Q96C36 | details |
| L-xylulose reductase | DCXR | 17q25.3 | Q7Z4W1 | details |
| Glucose-6-phosphate 1-dehydrogenase | G6PD | Xq28 | P11413 | details |
| Aldo-keto reductase family 1 member C3 | AKR1C3 | 10p15-p14 | P42330 | details |
| Aldo-keto reductase family 1 member C1 | AKR1C1 | 10p15-p14 | Q04828 | details |
| Aldo-keto reductase family 1 member C2 | AKR1C2 | 10p15-p14 | P52895 | details |
| Glyoxylate reductase/hydroxypyruvate reductase | GRHPR | 9q12 | Q9UBQ7 | details |
| Methionine synthase reductase | MTRR | 5p15.31 | Q9UBK8 | details |
| Sepiapterin reductase | SPR | 2p14-p12 | P35270 | details |
| Estradiol 17-beta-dehydrogenase 2 | HSD17B2 | 16q24.1-q24.2 | P37059 | details |
| Estradiol 17-beta-dehydrogenase 8 | HSD17B8 | 6p21.3 | Q92506 | details |
| Trans-1,2-dihydrobenzene-1,2-diol dehydrogenase | DHDH | 19q13.3 | Q9UQ10 | details |
| Cholesterol 7-alpha-monooxygenase | CYP7A1 | 8q11-q12 | P22680 | details |
| Alcohol dehydrogenase [NADP(+)] | AKR1A1 | 1p33-p32 | P14550 | details |
| Aldehyde dehydrogenase family 3 member B2 | ALDH3B2 | 11q13 | P48448 | details |
| Aldehyde dehydrogenase family 3 member B1 | ALDH3B1 | 11q13 | P43353 | details |
| Dihydropteridine reductase | QDPR | 4p15.31 | P09417 | details |
| 7-dehydrocholesterol reductase | DHCR7 | 11q13.4 | Q9UBM7 | details |
| 3-oxo-5-beta-steroid 4-dehydrogenase | AKR1D1 | 7q32-q33 | P51857 | details |
| Glutamate dehydrogenase 2, mitochondrial | GLUD2 | Xq24-q25 | P49448 | details |
| GMP reductase 2 | GMPR2 | 14q12 | Q9P2T1 | details |
| GMP reductase 1 | GMPR | 6p23 | P36959 | details |
| Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | GAPDHS | 19q13.12 | O14556 | details |
| Glutamate dehydrogenase 1, mitochondrial | GLUD1 | 10q23.3 | P00367 | details |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 12p13 | P04406 | details |
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 | SRD5A2 | 2p23 | P31213 | details |
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 | SRD5A1 | 5p15 | P18405 | details |
| Biliverdin reductase A | BLVRA | 7p13 | P53004 | details |
| Flavin reductase (NADPH) | BLVRB | 19q13.1-q13.2 | P30043 | details |
| Corticosteroid 11-beta-dehydrogenase isozyme 2 | HSD11B2 | 16q22 | P80365 | details |
| Corticosteroid 11-beta-dehydrogenase isozyme 1 | HSD11B1 | 1q32-q41 | P28845 | details |
| Dihydropyrimidine dehydrogenase [NADP(+)] | DPYD | 1p22 | Q12882 | details |
| Alcohol dehydrogenase class-3 | ADH5 | 4q23 | P11766 | details |
| Alcohol dehydrogenase 1B | ADH1B | 4q23 | P00325 | details |
| Alcohol dehydrogenase class 4 mu/sigma chain | ADH7 | 4q23-q24 | P40394 | details |
| Alcohol dehydrogenase 1A | ADH1A | 4q23 | P07327 | details |
| Alcohol dehydrogenase 6 | ADH6 | 4q23 | P28332 | details |
| Alcohol dehydrogenase 1C | ADH1C | 4q23 | P00326 | details |
| Dihydrofolate reductase | DHFR | 5q11.2-q13.2 | P00374 | details |
| Testosterone 17-beta-dehydrogenase 3 | HSD17B3 | 9q22 | P37058 | details |
| Estradiol 17-beta-dehydrogenase 1 | HSD17B1 | 17q11-q21 | P14061 | details |
| 3-keto-steroid reductase | HSD17B7 | 1q23 | P56937 | details |
| Alpha-aminoadipic semialdehyde synthase, mitochondrial | AASS | 7q31.3 | Q9UDR5 | details |
| Delta-1-pyrroline-5-carboxylate synthase | ALDH18A1 | 10q24.3 | P54886 | details |
| Isocitrate dehydrogenase [NADP], mitochondrial | IDH2 | 15q26.1 | P48735 | details |
| Isocitrate dehydrogenase [NADP] cytoplasmic | IDH1 | 2q33.3 | O75874 | details |
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase | HMGCR | 5q13.3-q14 | P04035 | details |
| NAD(P) transhydrogenase, mitochondrial | NNT | 5p12 | Q13423 | details |
| C-1-tetrahydrofolate synthase, cytoplasmic | MTHFD1 | 14q24 | P11586 | details |
| GDP-L-fucose synthase | TSTA3 | 8q24.3 | Q13630 | details |
| Nitric oxide synthase, inducible | NOS2 | 17q11.2-q12 | P35228 | details |
| Nitric oxide synthase, brain | NOS1 | 12q24.2-q24.31 | P29475 | details |
| Nitric oxide synthase, endothelial | NOS3 | 7q36 | P29474 | details |
| Cytosolic 10-formyltetrahydrofolate dehydrogenase | ALDH1L1 | 3q21.3 | O75891 | details |
| Cysteine dioxygenase type 1 | CDO1 | 5q23.2 | Q16878 | details |
| Trans-2-enoyl-CoA reductase, mitochondrial | MECR | 1p35.3 | Q9BV79 | details |
| Peroxisomal trans-2-enoyl-CoA reductase | PECR | 2q35 | Q9BY49 | details |
| Leukotriene-B(4) omega-hydroxylase 1 | CYP4F2 | 19p13.12 | P78329 | details |
| Leukotriene-B(4) omega-hydroxylase 2 | CYP4F3 | 19p13.2 | Q08477 | details |
| Sterol 26-hydroxylase, mitochondrial | CYP27A1 | 2q33-qter | Q02318 | details |
| 6-phosphogluconate dehydrogenase, decarboxylating | PGD | 1p36.22 | P52209 | details |
| 2,4-dienoyl-CoA reductase, mitochondrial | DECR1 | 8q21.3 | Q16698 | details |
| Quinone oxidoreductase | CRYZ | 1p31.1 | Q08257 | details |
| 25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial | CYP27B1 | 12q13.1-q13.3 | O15528 | details |
| Steroid 17-alpha-hydroxylase/17,20 lyase | CYP17A1 | 10q24.3 | P05093 | details |
| Peroxisomal 2,4-dienoyl-CoA reductase | DECR2 | 16p13.3 | Q9NUI1 | details |
| Neutrophil cytosol factor 2 | NCF2 | 1q25 | P19878 | details |
| Neutrophil cytosol factor 4 | NCF4 | 22q13.1 | Q15080 | details |
| 25-hydroxycholesterol 7-alpha-hydroxylase | CYP7B1 | 8q21.3 | O75881 | details |
| NAD(P)H dehydrogenase [quinone] 1 | NQO1 | 16q22.1 | P15559 | details |
| Glutaredoxin-1 | GLRX | 5q14 | P35754 | details |
| 1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial | CYP24A1 | 20q13 | Q07973 | details |
| Putative dimethylaniline monooxygenase [N-oxide-forming] 6 | FMO6P | | O60774 | details |
| Delta(24)-sterol reductase | DHCR24 | 1p32.3 | Q15392 | details |
| Neutrophil cytosol factor 1 | NCF1 | | P14598 | details |
| Kynurenine 3-monooxygenase | KMO | 1q42-q44 | O15229 | details |
| Dehydrogenase/reductase SDR family member 9 | DHRS9 | 2q31.1 | Q9BPW9 | details |
| Vitamin D 25-hydroxylase | CYP2R1 | 11p15.2 | Q6VVX0 | details |
| 17-beta-hydroxysteroid dehydrogenase type 6 | HSD17B6 | 12q13 | O14756 | details |
| Pyrroline-5-carboxylate reductase 3 | PYCRL | 8q24.3 | Q53H96 | details |
| Retinol dehydrogenase 12 | RDH12 | 14q24.1 | Q96NR8 | details |
| 3-ketodihydrosphingosine reductase | KDSR | 18q21.3 | Q06136 | details |
| Dual oxidase 2 | DUOX2 | 15q15.3 | Q9NRD8 | details |
| Aflatoxin B1 aldehyde reductase member 2 | AKR7A2 | 1p36.13 | O43488 | details |
| 3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase type 2 | HSD3B2 | 1p13.1 | P26439 | details |
| Cholesterol 24-hydroxylase | CYP46A1 | 14q32.1 | Q9Y6A2 | details |
| Retinol dehydrogenase 8 | RDH8 | 19p13.2 | Q9NYR8 | details |
| Retinol dehydrogenase 14 | RDH14 | 2p24.2 | Q9HBH5 | details |
| Retinol dehydrogenase 11 | RDH11 | 14q24.1 | Q8TC12 | details |
| 24-hydroxycholesterol 7-alpha-hydroxylase | CYP39A1 | 6p21.1-p11.2 | Q9NYL5 | details |
| 7-alpha-hydroxycholest-4-en-3-one 12-alpha-hydroxylase | CYP8B1 | 3p22.1 | Q9UNU6 | details |
| Retinol dehydrogenase 10 | RDH10 | 8q21.11 | Q8IZV5 | details |
| Dual oxidase 1 | DUOX1 | 15q15.3 | Q9NRD9 | details |
| Thioredoxin reductase 3 | TXNRD3 | 3q21.3 | Q86VQ6 | details |
| Mitochondrial 10-formyltetrahydrofolate dehydrogenase | ALDH1L2 | 12q23.3 | Q3SY69 | details |
| Fatty acyl-CoA reductase 1 | FAR1 | 11p15.2 | Q8WVX9 | details |
| Fatty acyl-CoA reductase 2 | FAR2 | 12p11.22 | Q96K12 | details |
| Putative uncharacterized protein DKFZp686B0215 | DKFZp686B0215 | | Q5HYI4 | details |
| Monofunctional C1-tetrahydrofolate synthase, mitochondrial | MTHFD1L | 6q25.1 | Q6UB35 | details |
| Acyl-CoA synthetase family member 4 | AASDH | 4q12 | Q4L235 | details |
| Aminoadipate-semialdehyde synthase | AASS | 7q31.3 | A4D0W4 | details |
| Putative uncharacterized protein PGD | PGD | 1p36.3-p36.13 | A9Z1X1 | details |
| Cytochrome P450, family 1, subfamily A, polypeptide 1 | CYP1A1 | 15q24.1 | A0N0X8 | details |
| NADPH oxidase 5 | NOX5 | 15q23 | Q96PH1 | details |
| NADPH oxidase 3 | NOX3 | 6q25.3 | Q9HBY0 | details |
| Protein-methionine sulfoxide oxidase MICAL1 | MICAL1 | 6q21 | Q8TDZ2 | details |
| Protein-methionine sulfoxide oxidase MICAL2 | MICAL2 | 11p15.3 | O94851 | details |
| Protein-methionine sulfoxide oxidase MICAL3 | MICAL3 | 22q11.21 | Q7RTP6 | details |
| NADPH oxidase 4 | NOX4 | 11q14.2-q21 | Q9NPH5 | details |
| Iodotyrosine dehalogenase 1 | IYD | 6q25.1 | Q6PHW0 | details |
| Peroxisomal coenzyme A diphosphatase NUDT7 | NUDT7 | 16q23.1 | P0C024 | details |
| Estradiol 17-beta-dehydrogenase 12 | HSD17B12 | 11p11.2 | Q53GQ0 | details |
| Prostaglandin reductase 2 | PTGR2 | 14q24.3 | Q8N8N7 | details |
| Short-chain dehydrogenase/reductase 3 | DHRS3 | 1p36.1 | O75911 | details |
| Oxidoreductase HTATIP2 | HTATIP2 | 11p15.1 | Q9BUP3 | details |
| Putative neutrophil cytosol factor 1B | NCF1B | 7q11.23 | A6NI72 | details |
| Putative neutrophil cytosol factor 1C | NCF1C | 7q11.23 | A8MVU1 | details |
| NADPH oxidase activator 1 | NOXA1 | 9q34.3 | Q86UR1 | details |
| Polyprenol reductase | SRD5A3 | 4q12 | Q9H8P0 | details |
| Very-long-chain enoyl-CoA reductase | TECR | 19p13.12 | Q9NZ01 | details |
| Cytochrome P450, family 21, subfamily A, polypeptide 2 | CYP21A2 | 6p21.3 | Q08AG9 | details |
| NOS1 mutant | NOS1 | 12q24.2-q24.31 | B3VK56 | details |
| L-aminoadipate-semialdehyde dehydrogenase-phosphopantetheinyl transferase | AASDHPPT | 11q22 | Q9NRN7 | details |
| Aldo-keto reductase family 1 member B10 | AKR1B10 | | O60218 | details |
| 1,5-anhydro-D-fructose reductase | AKR1E2 | 10p15.1 | Q96JD6 | details |
| Steroid 21-hydroxylase | CYP21A2 | 6p21.3 | P08686 | details |
| Dihydrofolate reductase, mitochondrial | DHFRL1 | 3q11.1 | Q86XF0 | details |
| ATP-dependent (S)-NAD(P)H-hydrate dehydratase | CARKD | 13q34 | Q8IW45 | details |