| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-04-17 18:42:00 UTC |
|---|
| Updated at | 2020-11-18 16:38:49 UTC |
|---|
| CannabisDB ID | CDB004838 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | Uridine 5'-monophosphate |
|---|
| Description | Uridine 5'-monophosphate, also known as uridylic acid or uridylate, belongs to the class of organic compounds known as pyrimidine ribonucleoside monophosphates. These are pyrimidine ribobucleotides with monophosphate group linked to the ribose moiety. Uridine 5'-monophosphate is an extremely weak basic (essentially neutral) compound (based on its pKa). Uridine 5'-monophosphate exists in all living species, ranging from bacteria to humans. Within humans, uridine 5'-monophosphate participates in a number of enzymatic reactions. In particular, uridine 5'-monophosphate can be converted into uridine 5'-diphosphate; which is catalyzed by the enzyme UMP-CMP kinase. In addition, uridine 5'-monophosphate can be biosynthesized from uridine 5'-diphosphate; which is catalyzed by the enzyme soluble calcium-activated nucleotidase 1. In humans, uridine 5'-monophosphate is involved in the metabolic disorder called the glut-1 deficiency syndrome pathway. Outside of the human body, Uridine 5'-monophosphate has been detected, but not quantified in, several different foods, such as naranjilla, cereals and cereal products, swedes, jicama, and chives. This could make uridine 5'-monophosphate a potential biomarker for the consumption of these foods. A pyrimidine ribonucleoside 5'-monophosphate having uracil as the nucleobase. Uridine 5'-monophosphate is expected to be in Cannabis as all living plants are known to produce and metabolize it. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5'-UMP | ChEBI | | 5'Uridylic acid | ChEBI | | pU | ChEBI | | UMP | ChEBI | | Uridine 5'-(dihydrogen phosphate) | ChEBI | | Uridine 5'-phosphate | ChEBI | | Uridine 5'-phosphoric acid | ChEBI | | Uridine monophosphate | ChEBI | | URIDINE-5'-monophosphATE | ChEBI | | Uridylate | ChEBI | | Uridylic acid | ChEBI | | 5'Uridylate | Generator | | Uridine 5'-(dihydrogen phosphoric acid) | Generator | | Uridine monophosphoric acid | Generator | | URIDINE-5'-monophosphoric acid | Generator | | Uridine 5'-monophosphoric acid | Generator | | Uridine 5'-phosphorate | HMDB | | Uridine mono(dihydrogen phosphate) | HMDB | | Uridine phosphate | HMDB | | Acids, uridylic | HMDB | | monoPhosphate, uridine | HMDB | | 5'-monoPhosphate, uridine | HMDB | | Uridylic acids | HMDB | | Acid, uridylic | HMDB | | Uridine 5' monophosphate | HMDB |
|
|---|
| Chemical Formula | C9H13N2O9P |
|---|
| Average Molecular Weight | 324.18 |
|---|
| Monoisotopic Molecular Weight | 324.0359 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid |
|---|
| Traditional Name | uridine monophosphate |
|---|
| CAS Registry Number | 58-97-9 |
|---|
| SMILES | O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(O)(O)=O)N1C=CC(=O)NC1=O |
|---|
| InChI Identifier | InChI=1S/C9H13N2O9P/c12-5-1-2-11(9(15)10-5)8-7(14)6(13)4(20-8)3-19-21(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,10,12,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 |
|---|
| InChI Key | DJJCXFVJDGTHFX-XVFCMESISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyrimidine ribonucleoside monophosphates. These are pyrimidine ribobucleotides with monophosphate group linked to the ribose moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Pyrimidine nucleotides |
|---|
| Sub Class | Pyrimidine ribonucleotides |
|---|
| Direct Parent | Pyrimidine ribonucleoside monophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Pentose monosaccharide
- Pyrimidone
- Monoalkyl phosphate
- Hydropyrimidine
- Monosaccharide
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Vinylogous amide
- Heteroaromatic compound
- Tetrahydrofuran
- 1,2-diol
- Urea
- Secondary alcohol
- Lactam
- Azacycle
- Oxacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Alcohol
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Industrial application: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | logP | Not Available | Not Available |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| GC-MS | Uridine 5'-monophosphate, 5 TMS, GC-MS Spectrum | splash10-014i-2953000000-f54596d9a11bbe82adc5 | Spectrum | | GC-MS | Uridine 5'-monophosphate, non-derivatized, GC-MS Spectrum | splash10-014i-2953000000-f54596d9a11bbe82adc5 | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-002b-9611000000-d241a590ba092a9137ca | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, 2 TMS, Predicted GC-MS Spectrum - 70eV, Positive | splash10-0fza-5933100000-760b9e44040ca55554b3 | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TMS_1_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TMS_1_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TMS_1_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TMS_1_4, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TMS_2_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TMS_2_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TMS_2_4, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TMS_2_5, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TMS_2_6, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TMS_2_7, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TBDMS_1_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TBDMS_1_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TBDMS_1_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TBDMS_1_4, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TBDMS_2_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TBDMS_2_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TBDMS_2_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TBDMS_2_4, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Uridine 5'-monophosphate, TBDMS_2_5, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-03fr-0194000000-c8bedd9fabaccee15ef6 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-03di-0910000000-da7fb1ddd74c36aec368 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-03di-0900000000-b2c9787a3d982ecb858e | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-00ba-9102000000-f8b96709e62e0857c40a | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-00ba-9102000000-f8b96709e62e0857c40a | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-05bb-9632000000-b8ba3967a5a4fa269881 | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-004i-9400000000-3d07e2fe57474fcd3e4b | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-00di-5039000000-c23fa9bbd6fcb5cf53d9 | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-004j-9111000000-5b5b7e0858807d74c34a | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-00os-9700000000-963e2c76c3f98512da72 | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-01r2-9510000000-b934a101047c97c33c7e | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-00di-3019000000-1bc3f90d1e162da0bc3c | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-004j-9211000000-5a9399a9558a148f349e | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-004j-9111000000-47125abb177da73df355 | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-004j-9000000000-c462cab8b32dc6c50361 | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9010000000-f3876f614e314a89ace6 | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-ef91704ffebfee64c252 | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-004j-9100000000-585ad2bb25aaaee5854c | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9200000000-60d486fec926bde90d2c | 2021-09-20 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-1911000000-7963cbf4b5fd08b83e4b | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-5900000000-404805c9de28466c7072 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9800000000-5522394ff370886e5a5d | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03mi-8934000000-b1f27b01000758ca974d | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9100000000-c3e61932dae0623d3924 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-4db5b2019eff03b12956 | 2016-09-12 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, D2O, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, D2O, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Lactose Synthesis | 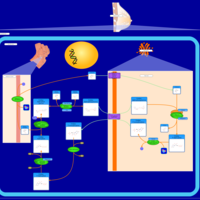 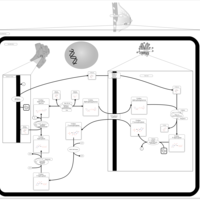 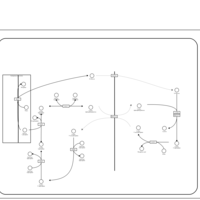 | Not Available | | Pyrimidine Metabolism |  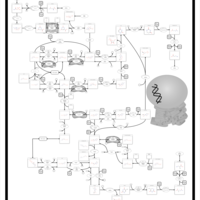  | 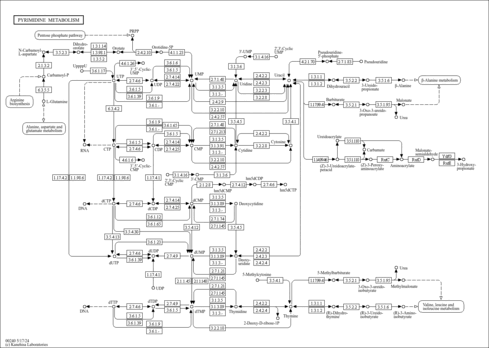 | | Transcription/Translation | Not Available | Not Available | | Congenital disorder of glycosylation CDG-IId |    | Not Available | | GLUT-1 deficiency syndrome | 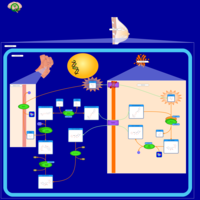 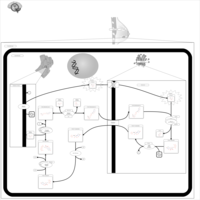 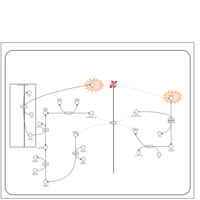 | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
|---|
| Transporters | Not Available |
|---|
| Metal Bindings | |
|---|
| Receptors | |
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 | ENPP1 | 6q22-q23 | P22413 | details | | Ectonucleotide pyrophosphatase/phosphodiesterase family member 3 | ENPP3 | 6q22 | O14638 | details |
|
|---|
| Transcriptional Factors | |
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta | GNPTAB | 12q23.2 | Q3T906 | details |
|
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0000288 |
|---|
| DrugBank ID | DB03685 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB031248 |
|---|
| KNApSAcK ID | C00007311 |
|---|
| Chemspider ID | 5808 |
|---|
| KEGG Compound ID | C00105 |
|---|
| BioCyc ID | UMP |
|---|
| BiGG ID | 33873 |
|---|
| Wikipedia Link | Uridine_monophosphate |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 6030 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 16695 |
|---|
| References |
|---|
| General References | Not Available |
|---|
