| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-04-17 18:45:24 UTC |
|---|
| Updated at | 2020-12-07 19:11:09 UTC |
|---|
| CannabisDB ID | CDB004871 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | L-Tryptophan |
|---|
| Description | L-Tryptophan, also known as Trp or W, belongs to the class of organic compounds known as indolyl carboxylic acids and derivatives. Indolyl carboxylic acids and derivatives are compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an indole ring. L-Tryptophan is a very strong basic compound (based on its pKa). L-Tryptophan exists in all living species, ranging from bacteria to humans. Within humans, L-tryptophan participates in a number of enzymatic reactions. In particular, L-tryptophan can be converted into n'-formylkynurenine through the action of the enzyme tryptophan 2,3-dioxygenase. In addition, L-tryptophan and tetrahydrobiopterin can be converted into 5-hydroxy-L-tryptophan and 4a-hydroxytetrahydrobiopterin through its interaction with the enzyme tryptophan 5-hydroxylase 1. The L-enantiomer of tryptophan. In humans, L-tryptophan is involved in tryptophan metabolism. Outside of the human body, L-Tryptophan is found, on average, in the highest concentration within a few different foods, such as caseins, steller sea lions, and evening primroses and in a lower concentration in thunnus, kefirs, and garden onions. L-Tryptophan has also been detected, but not quantified in, several different foods, such as thistles, gelatins, garden onion (var.), alpine sweetvetchs, and remoulades. This could make L-tryptophan a potential biomarker for the consumption of these foods. L-Tryptophan is a potentially toxic compound. L-Tryptophan is expected to be in Cannabis as all living plants are known to produce and metabolize it. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S)-2-Amino-3-(1H-indol-3-yl)propanoic acid | ChEBI | | (S)-alpha-Amino-1H-indole-3-propanoic acid | ChEBI | | (S)-alpha-Amino-beta-(3-indolyl)-propionic acid | ChEBI | | (S)-Tryptophan | ChEBI | | L-(-)-Tryptophan | ChEBI | | L-beta-3-Indolylalanine | ChEBI | | Trp | ChEBI | | Tryptophan | ChEBI | | W | ChEBI | | (2S)-2-Amino-3-(1H-indol-3-yl)propanoate | Generator | | (S)-a-Amino-1H-indole-3-propanoate | Generator | | (S)-a-Amino-1H-indole-3-propanoic acid | Generator | | (S)-alpha-Amino-1H-indole-3-propanoate | Generator | | (S)-Α-amino-1H-indole-3-propanoate | Generator | | (S)-Α-amino-1H-indole-3-propanoic acid | Generator | | (S)-a-Amino-b-(3-indolyl)-propionate | Generator | | (S)-a-Amino-b-(3-indolyl)-propionic acid | Generator | | (S)-alpha-Amino-beta-(3-indolyl)-propionate | Generator | | (S)-Α-amino-β-(3-indolyl)-propionate | Generator | | (S)-Α-amino-β-(3-indolyl)-propionic acid | Generator | | L-b-3-Indolylalanine | Generator | | L-Β-3-indolylalanine | Generator | | (-)-Tryptophan | HMDB | | (L)-Tryptophan | HMDB | | (S)-1H-Indole-3-alanine | HMDB | | (S)-2-Amino-3-(3-indolyl)propionic acid | HMDB | | (S)-a-Amino-b-indolepropionate | HMDB | | (S)-a-Amino-b-indolepropionic acid | HMDB | | (S)-a-Aminoindole-3-propionate | HMDB | | (S)-a-Aminoindole-3-propionic acid | HMDB | | (S)-alpha-Amino-beta-indolepropionate | HMDB | | (S)-alpha-Amino-beta-indolepropionic acid | HMDB | | (S)-alpha-Aminoindole-3-propionate | HMDB | | (S)-alpha-Aminoindole-3-propionic acid | HMDB | | 1-beta-3-Indolylalanine | HMDB | | 1beta-3-Indolylalanine | HMDB | | 1H-Indole-3-alanine | HMDB | | 2-Amino-3-indolylpropanoate | HMDB | | 2-Amino-3-indolylpropanoic acid | HMDB | | 3-(1H-indol-3-yl)-L-Alanine | HMDB | | 3-indol-3-Ylalanine | HMDB | | Alpha'-amino-3-indolepropionic acid | HMDB | | alpha-Aminoindole-3-propionic acid | HMDB | | Ardeytropin | HMDB | | H-TRP-OH | HMDB | | Indole-3-alanine | HMDB | | Kalma | HMDB | | L-alpha-Amino-3-indolepropionic acid | HMDB | | L-alpha-Aminoindole-3-propionic acid | HMDB | | L-Tryptofan | HMDB | | L-Tryptophane | HMDB | | Lopac-T-0254 | HMDB | | Lyphan | HMDB | | Optimax | HMDB | | Pacitron | HMDB | | Sedanoct | HMDB | | Triptofano | HMDB | | Trofan | HMDB | | Tryptacin | HMDB | | Tryptan | HMDB | | Tryptophane | HMDB | | Tryptophanum | HMDB | | Ardeydorm | HMDB | | L Tryptophan | HMDB | | L-Tryptophan-ratiopharm | HMDB | | Merck brand OF tryptophan | HMDB | | Niddapharm brand OF tryptophan | HMDB | | ICN brand OF tryptophan | HMDB | | Levotryptophan | HMDB | | PMS Tryptophan | HMDB | | PMS-Tryptophan | HMDB | | Ratiopharm brand OF tryptophan | HMDB | | Esparma brand OF tryptophan | HMDB | | Ratio-tryptophan | HMDB | | L Tryptophan ratiopharm | HMDB | | Naturruhe | HMDB | | Tryptophan metabolism alterations | HMDB | | Ardeypharm brand OF tryptophan | HMDB | | Kalma brand OF tryptophan | HMDB | | Pharmascience brand OF tryptophan | HMDB | | Upsher-smith brand OF tryptophan | HMDB | | Ratio tryptophan | HMDB |
|
|---|
| Chemical Formula | C11H12N2O2 |
|---|
| Average Molecular Weight | 204.23 |
|---|
| Monoisotopic Molecular Weight | 204.0899 |
|---|
| IUPAC Name | (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid |
|---|
| Traditional Name | L-tryptophan |
|---|
| CAS Registry Number | 73-22-3 |
|---|
| SMILES | N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m0/s1 |
|---|
| InChI Key | QIVBCDIJIAJPQS-VIFPVBQESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as indolyl carboxylic acids and derivatives. Indolyl carboxylic acids and derivatives are compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an indole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Indolyl carboxylic acids and derivatives |
|---|
| Direct Parent | Indolyl carboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indolyl carboxylic acid derivative
- Alpha-amino acid
- Alpha-amino acid or derivatives
- L-alpha-amino acid
- 3-alkylindole
- Indole
- Aralkylamine
- Benzenoid
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Amino acid or derivatives
- Amino acid
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Amine
- Primary aliphatic amine
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Physiological effect | Health effect: |
|---|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Indirect biological role: Industrial application: Biological role: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 230 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 13.4 mg/mL at 25 °C | YALKOWSKY,SH & DANNENFELSER,RM (1992) | | logP | -1.06 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| GC-MS | L-Tryptophan, 3 TMS, GC-MS Spectrum | splash10-0udi-0190000000-feaec8547634dddcad8c | Spectrum | | GC-MS | L-Tryptophan, 3 TMS, GC-MS Spectrum | splash10-0udi-0390000000-45a6c4fd79081597d44a | Spectrum | | GC-MS | L-Tryptophan, non-derivatized, GC-MS Spectrum | splash10-0udi-0290000000-34f7274f31a4cb321a0b | Spectrum | | GC-MS | L-Tryptophan, 3 TMS, GC-MS Spectrum | splash10-0fk9-9270000000-9761607cbe821f87f172 | Spectrum | | GC-MS | L-Tryptophan, 1 TMS, GC-MS Spectrum | splash10-001i-0900000000-3faeed7ad32e1755c03c | Spectrum | | GC-MS | L-Tryptophan, 3 TMS, GC-MS Spectrum | splash10-0udi-0290000000-9c57a732e337fade3cb6 | Spectrum | | GC-MS | L-Tryptophan, non-derivatized, GC-MS Spectrum | splash10-001i-0900000000-d054a214c1717940989f | Spectrum | | GC-MS | L-Tryptophan, non-derivatized, GC-MS Spectrum | splash10-0udi-0290000000-9860799c854e5c9ac1c7 | Spectrum | | GC-MS | L-Tryptophan, non-derivatized, GC-MS Spectrum | splash10-0udi-0190000000-feaec8547634dddcad8c | Spectrum | | GC-MS | L-Tryptophan, non-derivatized, GC-MS Spectrum | splash10-0udi-0390000000-45a6c4fd79081597d44a | Spectrum | | GC-MS | L-Tryptophan, non-derivatized, GC-MS Spectrum | splash10-0udi-0290000000-34f7274f31a4cb321a0b | Spectrum | | GC-MS | L-Tryptophan, non-derivatized, GC-MS Spectrum | splash10-0udi-3729000000-86129db57aaf1a245f93 | Spectrum | | GC-MS | L-Tryptophan, non-derivatized, GC-MS Spectrum | splash10-0fk9-9270000000-9761607cbe821f87f172 | Spectrum | | GC-MS | L-Tryptophan, non-derivatized, GC-MS Spectrum | splash10-001i-0900000000-3faeed7ad32e1755c03c | Spectrum | | GC-MS | L-Tryptophan, non-derivatized, GC-MS Spectrum | splash10-0udi-0290000000-9c57a732e337fade3cb6 | Spectrum | | Predicted GC-MS | L-Tryptophan, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-0560-4900000000-fa932bc4cffed0ca66b7 | Spectrum | | Predicted GC-MS | L-Tryptophan, 1 TMS, Predicted GC-MS Spectrum - 70eV, Positive | splash10-00e9-9560000000-6829a8b2a2096883999f | Spectrum | | Predicted GC-MS | L-Tryptophan, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | L-Tryptophan, TMS_1_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | L-Tryptophan, TMS_1_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | L-Tryptophan, TBDMS_1_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | L-Tryptophan, TBDMS_1_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | L-Tryptophan, TBDMS_1_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0udi-0290602010-c09c931538bff74ac400 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0a4i-0900000000-0fed327c2a56f556e04c | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0udi-0090000000-98ec1c2c012e58eab924 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-00di-0090000000-fc77784da5d9b288d751 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0udi-0290601010-d6f94902c0cf639cb7bc | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0a4i-0900000000-27633a4f7ecfac45c730 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0udi-0090000000-ee6cb8392b2e8d644bc1 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-004i-0090000000-d81c86eceee1c9824b02 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0udi-0190000000-0e8b883dc8ab06c89d77 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0uxu-2940000000-cb35b9680612e19d8b3a | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-014i-2900000000-7cc592351cc616b1d75f | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-014i-1900000000-987615a0add5eb2c3169 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-014i-1900000000-f150d9d1e19c72d337a8 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-0uxr-2960000000-f4c878f3abb1609bb5c5 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0udi-0090000000-c73c9b49139319c80b18 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0gb9-0930000000-da94834297fcc5b3d3c5 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-014i-0900000000-94061fd1f6b08445cdf3 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-014i-0900000000-e78172f4f27df232466e | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-014i-0900000000-93406e2cdb79770abc7d | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0udi-0190000000-0e8b883dc8ab06c89d77 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0uxu-2940000000-cb35b9680612e19d8b3a | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-014i-2900000000-5187dac98dbd6834d6ba | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-014i-1900000000-987615a0add5eb2c3169 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-014i-1900000000-174bb4d4390c75ae9a09 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0a4i-0900000000-0fed327c2a56f556e04c | 2017-09-14 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 125 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, experimental) | | Spectrum | | 2D NMR | [1H, 1H]-TOCSY. Unexported temporarily by An Chi on Oct 15, 2021 until json or nmrML file is generated. 2D NMR Spectrum (experimental) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Tryptophan Metabolism |  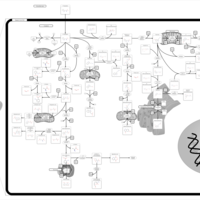  | 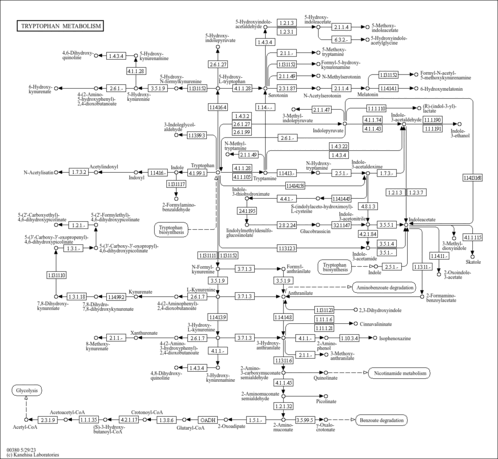 | | Transcription/Translation | Not Available | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
|---|
| Transporters | |
|---|
| Metal Bindings | |
|---|
| Receptors | Not Available |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0000929 |
|---|
| DrugBank ID | DB00150 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB002250 |
|---|
| KNApSAcK ID | C00001396 |
|---|
| Chemspider ID | 6066 |
|---|
| KEGG Compound ID | C00078 |
|---|
| BioCyc ID | TRP |
|---|
| BiGG ID | 33772 |
|---|
| Wikipedia Link | Tryptophan |
|---|
| METLIN ID | 5879 |
|---|
| PubChem Compound | 6305 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 16828 |
|---|
| References |
|---|
| General References | Not Available |
|---|
