| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-04-17 18:38:53 UTC |
|---|
| Updated at | 2020-12-07 19:10:59 UTC |
|---|
| CannabisDB ID | CDB004808 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | Guanosine |
|---|
| Description | Guanosine, also known as G or 2-amino-inosine, belongs to the class of organic compounds known as purine nucleosides. Purine nucleosides are compounds comprising a purine base attached to a ribosyl or deoxyribosyl moiety. Guanosine is an extremely weak basic (essentially neutral) compound (based on its pKa). Guanosine is the specific extracellular guanine-based purines effector and its conversion occurs not only in the central nervous system but also peripherally (PMID: 16325434 ). Guanosine exists in all living species, ranging from bacteria to humans. In humans, guanosine is involved in intracellular signalling through adenosine receptor A2B and adenosine. Guanosine is a potentially toxic compound. This nucleoside exerts important neuroprotective and neuromodulator roles in the central nervous system, which may be related to inhibition of the glutamatergic neurotransmission activity. Guanosine can be phosphorylated to become GMP (guanosine monophosphate), cGMP (cyclic guanosine monophosphate), GDP (guanosine diphosphate) and GTP (guanosine triphosphate) which are factors in signal transduction pathways. Guanosine can be phosphorylated to become GMP (guanosine monophosphate), cGMP (cyclic guanosine monophosphate), GDP (guanosine diphosphate), and GTP (guanosine triphosphate) (Wikipedia ). Guanosine is expected to be in Cannabis as all living plants are known to produce and metabolize it. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2(3H)-Imino-9-beta-D-ribofuranosyl-9H-purin-6(1H)-one | ChEBI | | 2-Amino-1,9-dihydro-9-beta-D-ribofuranosyl-6H-purin-6-one | ChEBI | | 2-Amino-9-beta-D-ribofuranosyl-1,9-dihydro-6H-purin-6-one | ChEBI | | 9-beta-D-Ribofuranosyl-guanine | ChEBI | | G | ChEBI | | Guanine riboside | ChEBI | | Guanine-9-beta-D-ribofuranoside | ChEBI | | Guanosin | ChEBI | | Guo | ChEBI | | 2(3H)-Imino-9-b-D-ribofuranosyl-9H-purin-6(1H)-one | Generator | | 2(3H)-Imino-9-β-D-ribofuranosyl-9H-purin-6(1H)-one | Generator | | 2-Amino-1,9-dihydro-9-b-D-ribofuranosyl-6H-purin-6-one | Generator | | 2-Amino-1,9-dihydro-9-β-D-ribofuranosyl-6H-purin-6-one | Generator | | 2-Amino-9-b-D-ribofuranosyl-1,9-dihydro-6H-purin-6-one | Generator | | 2-Amino-9-β-D-ribofuranosyl-1,9-dihydro-6H-purin-6-one | Generator | | 9-b-D-Ribofuranosyl-guanine | Generator | | 9-Β-D-ribofuranosyl-guanine | Generator | | Guanine-9-b-D-ribofuranoside | Generator | | Guanine-9-β-D-ribofuranoside | Generator | | 2-Amino-1,9-dihydro-9-beta-delta-ribofuranosyl-6H-purin-6-one | HMDB | | 2-Amino-inosine | HMDB | | 9-beta-delta-Ribofuranosyl-guanine | HMDB | | b-D-Ribofuranoside guanine-9 | HMDB | | beta-delta-Ribofuranoside guanine-9 | HMDB | | Ribonucleoside | HMDB | | Vernine | HMDB |
|
|---|
| Chemical Formula | C10H13N5O5 |
|---|
| Average Molecular Weight | 283.24 |
|---|
| Monoisotopic Molecular Weight | 283.0917 |
|---|
| IUPAC Name | 2-amino-9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-6,9-dihydro-1H-purin-6-one |
|---|
| Traditional Name | guanosine |
|---|
| CAS Registry Number | 118-00-3 |
|---|
| SMILES | NC1=NC2=C(N=CN2[C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)C(=O)N1 |
|---|
| InChI Identifier | InChI=1S/C10H13N5O5/c11-10-13-7-4(8(19)14-10)12-2-15(7)9-6(18)5(17)3(1-16)20-9/h2-3,5-6,9,16-18H,1H2,(H3,11,13,14,19)/t3-,5-,6-,9-/m1/s1 |
|---|
| InChI Key | NYHBQMYGNKIUIF-UUOKFMHZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as purine nucleosides. Purine nucleosides are compounds comprising a purine base attached to a ribosyl or deoxyribosyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Purine nucleosides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Purine nucleosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Purine nucleoside
- Glycosyl compound
- N-glycosyl compound
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Hydroxypyrimidine
- Monosaccharide
- N-substituted imidazole
- Pyrimidine
- Heteroaromatic compound
- Azole
- Imidazole
- Tetrahydrofuran
- Secondary alcohol
- Azacycle
- Oxacycle
- Organoheterocyclic compound
- Primary alcohol
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Physiological effect | Health effect: |
|---|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Industrial application: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 239 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.7 mg/mL at 18 °C | Not Available | | logP | -1.90 | SANGSTER (1993) |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| EI-MS | Mass Spectrum (Electron Ionization) | splash10-0udl-9400000000-5a915ef7129c50e638a7 | 2018-05-25 | View Spectrum | | GC-MS | Guanosine, 5 TMS, GC-MS Spectrum | splash10-0f7k-1952000000-c57c052c65f3fa9504af | Spectrum | | GC-MS | Guanosine, non-derivatized, GC-MS Spectrum | splash10-0feb-0963000000-c91b93f300b50fbc99c1 | Spectrum | | GC-MS | Guanosine, 4 TMS, GC-MS Spectrum | splash10-00di-9440000000-361092fc2dc5206fe655 | Spectrum | | GC-MS | Guanosine, 5 TMS, GC-MS Spectrum | splash10-00di-9421000000-b2b15c03b8206196f59f | Spectrum | | GC-MS | Guanosine, non-derivatized, GC-MS Spectrum | splash10-0f7k-1952000000-c57c052c65f3fa9504af | Spectrum | | GC-MS | Guanosine, non-derivatized, GC-MS Spectrum | splash10-0feb-0963000000-c91b93f300b50fbc99c1 | Spectrum | | GC-MS | Guanosine, non-derivatized, GC-MS Spectrum | splash10-00di-9440000000-361092fc2dc5206fe655 | Spectrum | | GC-MS | Guanosine, non-derivatized, GC-MS Spectrum | splash10-00di-9421000000-b2b15c03b8206196f59f | Spectrum | | Predicted GC-MS | Guanosine, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-0zml-9670000000-793c36e66ed6430b932b | Spectrum | | Predicted GC-MS | Guanosine, 3 TMS, Predicted GC-MS Spectrum - 70eV, Positive | splash10-0159-4945800000-86e8114119c47d85cc53 | Spectrum | | Predicted GC-MS | Guanosine, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_1_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_1_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_1_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_1_4, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_1_5, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_2_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_2_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_2_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_2_4, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_2_5, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_2_6, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_2_7, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Guanosine, TMS_2_8, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0udi-0900000000-ef0cae7d67f9e803f2b2 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0udi-0900000000-68fb606342b8bb123bb7 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0f89-0900000000-48d32b9d62eda760619e | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0f7k-0920000000-f3db23cc762b5fb860e4 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0900000000-5da62630f03e15d8707c | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0900000000-1fa2eb473b57a37ecd45 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0udi-0900000000-d71a3d2995d32c692eb7 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0390000000-ff54fe98a9c0e1bc104c | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0900000000-c023445b0f47e6e7267b | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0udi-0900000000-a094aa75c261cdce24de | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0udi-0900000000-e114cc69f7ab438abb39 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001j-0795222100-55ad26c7c4d9f64dd759 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-004i-0900000000-9450dc04b32ca468e146 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0002-0900000000-0147dd895ffbecfa9668 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-03di-0190000000-5d00bb466dd23f062b86 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0f89-0690030010-86fae3a6cae3e2b3de2c | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001i-0900000000-97217b0084eeac14e7e1 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0udi-0900000000-d0c65e36192b7ebdaae8 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001i-0090000000-bb8ef433eba795395fe3 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-001i-0090000000-4ceff605ca97b7c0774a | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0ue9-0960000000-1c286e55d437f33a29b3 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0udi-0900000000-7149427a8c09f429fdbe | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0zgi-0900000000-5de849ca7144ca8afe05 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-053r-0900000000-f97dbc10f152af4df778 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-0ue9-0970000000-045435f1be487a94195c | 2012-08-31 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 125 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | | Spectrum | | 2D NMR | [1H, 1H]-TOCSY. Unexported temporarily by An Chi on Oct 15, 2021 until json or nmrML file is generated. 2D NMR Spectrum (experimental) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 400 MHz, H2O, experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Purine Metabolism |  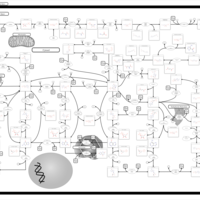 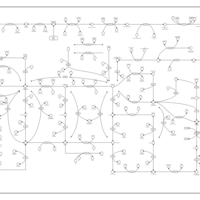 |  | | Adenosine Deaminase Deficiency |  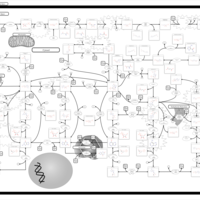 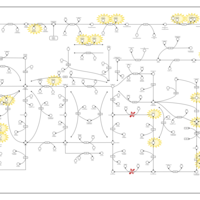 | Not Available | | Adenylosuccinate Lyase Deficiency | 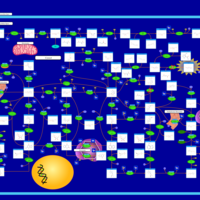 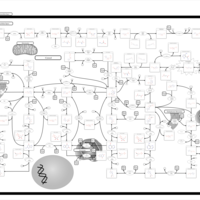 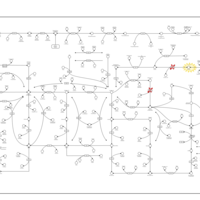 | Not Available | | Gout or Kelley-Seegmiller Syndrome | 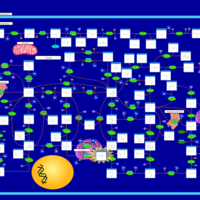 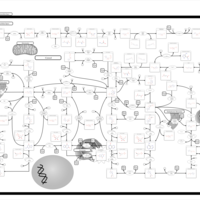 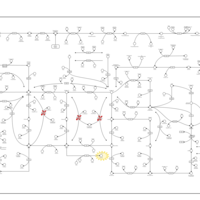 | Not Available | | Lesch-Nyhan Syndrome (LNS) | 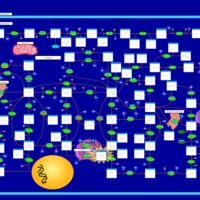 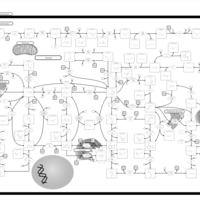 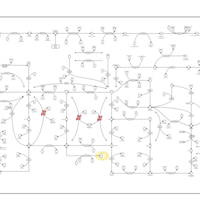 | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
|---|
| Transporters | Not Available |
|---|
| Metal Bindings | |
|---|
| Receptors | Not Available |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0000133 |
|---|
| DrugBank ID | DB02857 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB003632 |
|---|
| KNApSAcK ID | C00019679 |
|---|
| Chemspider ID | 6544 |
|---|
| KEGG Compound ID | C00387 |
|---|
| BioCyc ID | GUANOSINE |
|---|
| BiGG ID | 51567 |
|---|
| Wikipedia Link | Guanosine |
|---|
| METLIN ID | 87 |
|---|
| PubChem Compound | 6802 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 16750 |
|---|
| References |
|---|
| General References | - Saute JA, da Silveira LE, Soares FA, Martini LH, Souza DO, Ganzella M: Amnesic effect of GMP depends on its conversion to guanosine. Neurobiol Learn Mem. 2006 May;85(3):206-12. doi: 10.1016/j.nlm.2005.10.006. Epub 2005 Dec 1. [PubMed:16325434 ]
|
|---|
