| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-04-17 19:07:55 UTC |
|---|
| Updated at | 2020-12-07 19:11:32 UTC |
|---|
| CannabisDB ID | CDB005086 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | Glyceric acid 1,3-biphosphate |
|---|
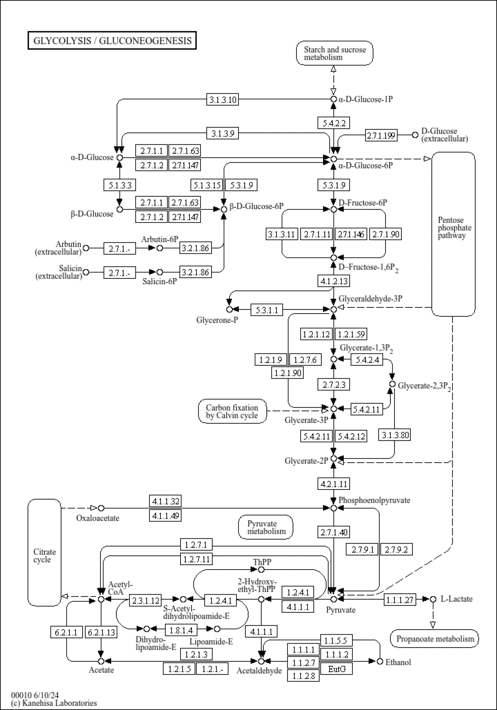
| Description | Glyceric acid 1,3-biphosphate, also known as 1,3-bisphospho-D-glycerate or 3-phospho-D-glyceroyl phosphate, belongs to the class of organic compounds known as acyl monophosphates. These are organic compounds containing a monophosphate linked to an acyl group. They have the general structure R-CO-P(O)(O)OH, R=H or organyl. The (R)-enantiomer of 3-phosphoglyceroyl dihydrogen phosphate. Glyceric acid 1,3-biphosphate is an extremely weak basic (essentially neutral) compound (based on its pKa). Glyceric acid 1,3-biphosphate exists in all living species, ranging from bacteria to humans. Within humans, glyceric acid 1,3-biphosphate participates in a number of enzymatic reactions. In particular, glyceric acid 1,3-biphosphate can be biosynthesized from D-glyceraldehyde 3-phosphate; which is catalyzed by the enzyme glyceraldehyde-3-phosphate dehydrogenase. In addition, glyceric acid 1,3-biphosphate can be biosynthesized from 3-phosphoglyceric acid through the action of the enzyme pantothenate kinase 1. In humans, glyceric acid 1,3-biphosphate is involved in the metabolic disorder called the glycogen storage disease type 1A (gsd1a) or von gierke disease pathway. Outside of the human body, Glyceric acid 1,3-biphosphate has been detected, but not quantified in, several different foods, such as cherry tomato, jostaberries, canada blueberries, lovages, and mamey sapotes. This could make glyceric acid 1,3-biphosphate a potential biomarker for the consumption of these foods. Glyceric acid 1,3-biphosphate is expected to be in Cannabis as all living plants are known to produce and metabolize it. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R)-2-Hydroxy-3-(phosphonooxy)-1-monoanhydride with phosphoric propanoic acid | ChEBI | | 1,3-Bisphospho-D-glycerate | ChEBI | | 3-Phospho-D-glyceroyl phosphate | ChEBI | | D-Glycerate 1,3-diphosphate | Kegg | | (R)-2-Hydroxy-3-(phosphonooxy)-1-monoanhydride with phosphoric propanoate | Generator | | 1,3-Bisphospho-D-glyceric acid | Generator | | 3-Phospho-D-glyceroyl phosphoric acid | Generator | | D-Glyceric acid 1,3-diphosphoric acid | Generator | | Glycerate 1,3-biphosphate | Generator | | Glyceric acid 1,3-biphosphoric acid | Generator | | 3-Phospho-D-glyceroyl dihydrogen phosphoric acid | HMDB | | 1,3-Biphosphoglycerate | HMDB | | 1,3-Bisphosphoglycerate | HMDB | | 1,3-Bisphosphoglyceric acid | HMDB | | 1,3-Diphosphoglycerate | HMDB | | 1,3-Diphosphoglyceric acid | HMDB | | 3-Phospho-D-glyceroyl-phosphate | HMDB | | 3-Phosphoglyceroyl phosphate | HMDB | | 3-Phosphoglyceroyl-p | HMDB | | 3-Phosphoglyceroyl-phosphate | HMDB | | 3-Phosphonato-D-glyceroyl phosphate | HMDB | | 3-Phosphonatoglyceroyl phosphate | HMDB | | 3-p-Glyceroyl-p | HMDB | | D-Glycerate 1,3-biphosphate | HMDB | | D-Glycerate 1,3-bisphosphate | HMDB | | D-Glyceric acid 1,3-biphosphate | HMDB | | D-Glyceric acid 1,3-bisphosphate | HMDB | | D-Glyceric acid 1,3-diphosphate | HMDB | | DPG | HMDB | | Glycerate 1,3-bisphosphate | HMDB | | Glycerate 1,3-diphosphate | HMDB | | Glyceric acid 1,3-bisphosphate | HMDB | | Glyceric acid 1,3-diphosphate | HMDB | | Phosphoglyceroyl-p | HMDB | | p-Glyceroyl-p | HMDB | | Glyceric acid 1,3-biphosphate | HMDB |
|
|---|
| Chemical Formula | C3H8O10P2 |
|---|
| Average Molecular Weight | 266.04 |
|---|
| Monoisotopic Molecular Weight | 265.9593 |
|---|
| IUPAC Name | {[(2R)-2-hydroxy-3-(phosphonooxy)propanoyl]oxy}phosphonic acid |
|---|
| Traditional Name | 1,3-bisphospho-D-glycerate |
|---|
| CAS Registry Number | 38168-82-0 |
|---|
| SMILES | O[C@H](COP(O)(O)=O)C(=O)OP(O)(O)=O |
|---|
| InChI Identifier | InChI=1S/C3H8O10P2/c4-2(1-12-14(6,7)8)3(5)13-15(9,10)11/h2,4H,1H2,(H2,6,7,8)(H2,9,10,11)/t2-/m1/s1 |
|---|
| InChI Key | LJQLQCAXBUHEAZ-UWTATZPHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as acyl monophosphates. These are organic compounds containing a monophosphate linked to an acyl group. They have the general structure R-CO-P(O)(O)OH, R=H or organyl. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic phosphoric acids and derivatives |
|---|
| Sub Class | Phosphate esters |
|---|
| Direct Parent | Acyl monophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyl monophosphate
- Glyceric_acid
- Monoalkyl phosphate
- Monosaccharide
- Alkyl phosphate
- Carboxylic acid salt
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic salt
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Physiological effect | Health effect: |
|---|
| Disposition | Source: Biological location: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | logP | Not Available | Not Available |
|
|---|
| Predicted Properties | [] |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Glyceric acid 1,3-biphosphate, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-0002-9500000000-cb3071f9249ee92b7d2c | Spectrum | | Predicted GC-MS | Glyceric acid 1,3-biphosphate, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-1950000000-2cde7b5739871a882542 | 2015-09-15 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014m-3910000000-72f2c46eab2036938056 | 2015-09-15 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-9500000000-c835034f0ac11618922d | 2015-09-15 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03fr-6190000000-6f4870543fc6131d14a7 | 2015-09-15 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-d9d9c6d217a255d791fd | 2015-09-15 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-1fef400a1ea8ca54c802 | 2015-09-15 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03dj-0090000000-def40aa9bb3094302ce8 | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01r2-9040000000-374a9ff8c866f10f2f3c | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-4bf2b9a14f4e1c8fc62a | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-1390000000-8fe62bbfc56bf59f28a6 | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9410000000-e17767e21640a6f81532 | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-c41dab2684b9d54afd31 | 2021-09-22 | View Spectrum |
|
|---|