Showing Compound Card for 3-Hydroxy-3-methylglutaryl-CoA (CDB004909)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Created at | 2020-04-17 18:49:25 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Updated at | 2020-12-07 19:11:15 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CannabisDB ID | CDB004909 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cannabis Compound Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | 3-Hydroxy-3-methylglutaryl-CoA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | 3-Hydroxy-3-methylglutaryl-CoA, also known as hydroxymethylglutaroyl coenzyme A or HMG-CoA, belongs to the class of organic compounds known as (s)-3-hydroxy-3-alkylglutaryl coas. These are 3-hydroxy-3-alkylglutaryl-CoAs where the 3-hydroxy-3-alkylglutaryl component has (S)-configuration. Thus, 3-hydroxy-3-methylglutaryl-CoA is considered to be a fatty ester lipid molecule. 3-Hydroxy-3-methylglutaryl-CoA is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. 3-Hydroxy-3-methylglutaryl-CoA is expected to be in Cannabis as all living plants are known to produce and metabolize it. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C27H44N7O20P3S | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight | 911.66 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight | 911.1575 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | (3S)-5-[(2-{3-[(2R)-3-[({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-2-hydroxy-3-methylbutanamido]propanamido}ethyl)sulfanyl]-3-hydroxy-3-methyl-5-oxopentanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | HMG-CoA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 26926-09-0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | C[C@](O)(CC(O)=O)CC(=O)SCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C27H44N7O20P3S/c1-26(2,21(40)24(41)30-5-4-15(35)29-6-7-58-17(38)9-27(3,42)8-16(36)37)11-51-57(48,49)54-56(46,47)50-10-14-20(53-55(43,44)45)19(39)25(52-14)34-13-33-18-22(28)31-12-32-23(18)34/h12-14,19-21,25,39-40,42H,4-11H2,1-3H3,(H,29,35)(H,30,41)(H,36,37)(H,46,47)(H,48,49)(H2,28,31,32)(H2,43,44,45)/t14-,19-,20-,21+,25-,27+/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | CABVTRNMFUVUDM-VRHQGPGLSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Belongs to the class of organic compounds known as (s)-3-hydroxy-3-alkylglutaryl coas. These are 3-hydroxy-3-alkylglutaryl-CoAs where the 3-hydroxy-3-alkylglutaryl component has (S)-configuration. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Fatty Acyls | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Fatty acyl thioesters | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | (S)-3-hydroxy-3-alkylglutaryl CoAs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ontology | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Disposition | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EI-MS/GC-MS | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MS/MS |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
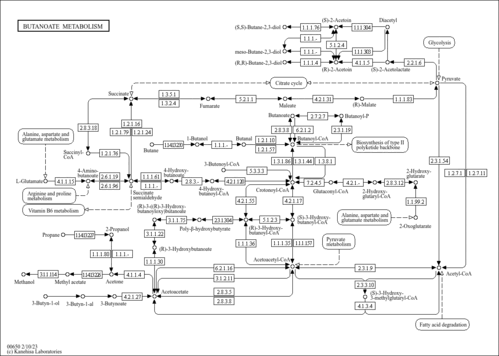
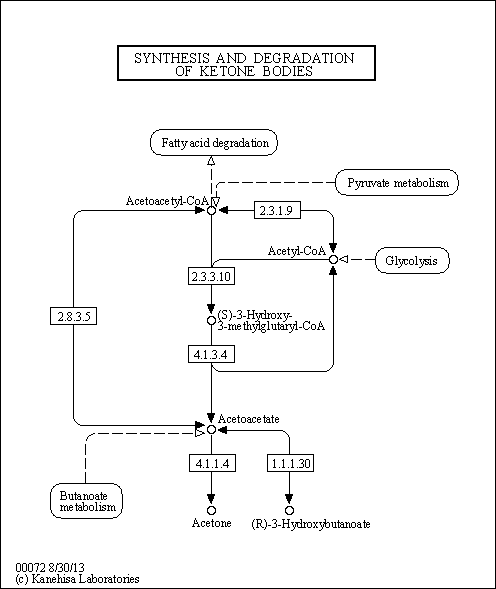
| Pathways | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Protein Targets | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Enzymes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Transporters | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metal Bindings |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Receptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Transcriptional Factors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations Data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB0001375 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenol Explorer Compound ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FoodDB ID | FDB030158 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KNApSAcK ID | C00007270 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemspider ID | 392859 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Compound ID | C00356 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | 3-HYDROXY-3-METHYL-GLUTARYL-COA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BiGG ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| METLIN ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound | 445127 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 15467 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- Key enzyme in ketogenesis (ketone body formation). Terminal step in leucine catabolism.
- Gene Name:
- HMGCL
- Uniprot ID:
- P35914
- Molecular weight:
- 34359.84
- General function:
- Involved in hydroxymethylglutaryl-CoA synthase activity
- Specific function:
- This enzyme condenses acetyl-CoA with acetoacetyl-CoA to form HMG-CoA, which is the substrate for HMG-CoA reductase.
- Gene Name:
- HMGCS2
- Uniprot ID:
- P54868
- Molecular weight:
- 52481.065
- General function:
- Involved in hydroxymethylglutaryl-CoA synthase activity
- Specific function:
- This enzyme condenses acetyl-CoA with acetoacetyl-CoA to form HMG-CoA, which is the substrate for HMG-CoA reductase.
- Gene Name:
- HMGCS1
- Uniprot ID:
- Q01581
- Molecular weight:
- 57293.105
- General function:
- Involved in hydroxymethylglutaryl-CoA reductase (NADPH) activity
- Specific function:
- Transmembrane glycoprotein that is the rate-limiting enzyme in cholesterol biosynthesis as well as in the biosynthesis of nonsterol isoprenoids that are essential for normal cell function including ubiquinone and geranylgeranyl proteins.
- Gene Name:
- HMGCR
- Uniprot ID:
- P04035
- Molecular weight:
- 97475.155
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes the conversion of 3-methylglutaconyl-CoA to 3-hydroxy-3-methylglutaryl-CoA. Has very low enoyl-CoA hydratase activity. Was originally identified as RNA-binding protein that binds in vitro to clustered 5'-AUUUA-3' motifs.
- Gene Name:
- AUH
- Uniprot ID:
- Q13825
- Molecular weight:
- 35608.18
- General function:
- Involved in catalytic activity
- Specific function:
- Non-mitochondrial 3-hydroxymethyl-3-methylglutaryl-CoA lyase that catalyzes a cation-dependent cleavage of (S)-3-hydroxy-3-methylglutaryl-CoA into acetyl-CoA and acetoacetate, a key step in ketogenesis, the products of which support energy production in nonhepatic animal tissues.
- Gene Name:
- HMGCLL1
- Uniprot ID:
- Q8TB92
- Molecular weight:
- 36327.465