| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-03-30 17:43:57 UTC |
|---|
| Updated at | 2020-12-07 19:07:47 UTC |
|---|
| CannabisDB ID | CDB000805 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | PS(16:0/16:0) |
|---|
| Description | PS(16:0/16:0) is a phosphatidylserine. It is a glycerophospholipid in which a phosphorylserine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidylserines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PS(16:0/16:0), in particular, consists of two hexadecanoyl chains at positions C-1 and C-2. Phosphatidylserine or 1,2-diacyl-sn-glycero-3-phospho-L-serine is distributed widely among animals, plants and microorganisms. Phosphatidylserine is an acidic (anionic) phospholipid with three ionizable groups, i.e. the phosphate moiety, the amino group and the carboxyl function. As with other acidic lipids, it exists in nature in salt form, but it has a high propensity to chelate to calcium via the charged oxygen atoms of both the carboxyl and phosphate moieties, modifying the conformation of the polar head group. This interaction may be of considerable relevance to the biological function of phosphatidylserine. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. Phosphatidylserines typically carry a net charge of -1 at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PS biosynthesis involves an exchange reaction of serine for ethanolamine in PE. This compound is expected to be in Cannabis as all living plants are known to produce and metabolize it. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2-Dipalmitoyl-sn-glycero-3-phosphoserine | ChEBI | | 1,2-Dipalmitoylglycerophosphorylserine | ChEBI | | Phosphatidylserine (16:0/16:0) | ChEBI | | pSer(16:0/16:0) | ChEBI | | PS(32:0) | HMDB | | Phosphatidylserine(16:0/16:0) | HMDB | | 1,2-Dipalmitoyl-rac-glycero-3-phosphoserine | HMDB | | pSer(32:0) | HMDB | | 1,2-Dihexadecanoyl-rac-glycero-3-phosphoserine | HMDB | | Phosphatidylserine(32:0) | HMDB | | DPPS | HMDB | | Dipalmitoylphosphatidylserine | HMDB | | 1,2-Dipalmitoyl-GPS | HMDB | | 1,2-Dipalmitoyl-sn-3-glycerophosphoserine | HMDB | | 1,2-Dipalmitoyl-sn-glycero-3-phosphatidylserine | HMDB | | 1,2-Dipalmitoyl-sn-glycero-3-phospho-L-serine | HMDB | | Dipalmitoyl-sn-glycerol-3-phosphorylserine | HMDB | | L-alpha-Dipalmitoylphosphatidylserine | HMDB | | L-Α-dipalmitoylphosphatidylserine | HMDB | | PS(16:0/16:0) | Lipid Annotator, ChEBI |
|
|---|
| Chemical Formula | C38H74NO10P |
|---|
| Average Molecular Weight | 735.98 |
|---|
| Monoisotopic Molecular Weight | 735.505 |
|---|
| IUPAC Name | (2S)-2-amino-3-({[(2R)-2,3-bis(hexadecanoyloxy)propoxy](hydroxy)phosphoryl}oxy)propanoic acid |
|---|
| Traditional Name | dipalmitoyl phosphatidylserine |
|---|
| CAS Registry Number | 40290-42-4 |
|---|
| SMILES | CCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C38H74NO10P/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-36(40)46-31-34(32-47-50(44,45)48-33-35(39)38(42)43)49-37(41)30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h34-35H,3-33,39H2,1-2H3,(H,42,43)(H,44,45)/t34-,35+/m1/s1 |
|---|
| InChI Key | KLFKZIQAIPDJCW-GPOMZPHUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as phosphatidylserines. These are glycerophosphoserines in which two fatty acids are bonded to the glycerol moiety through ester linkages. As is the case with diacylglycerols, phosphatidylserines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoserines |
|---|
| Direct Parent | Phosphatidylserines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diacyl-glycerol-3-phosphoserine
- Alpha-amino acid
- Alpha-amino acid or derivatives
- L-alpha-amino acid
- Tricarboxylic acid or derivatives
- Phosphoethanolamine
- Fatty acid ester
- Dialkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Amino acid
- Amino acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Carboxylic acid
- Primary aliphatic amine
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Carbonyl group
- Organic oxide
- Primary amine
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Physiological effect | Organoleptic effect: |
|---|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Industrial application: Biological role: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | logP | Not Available | Not Available |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | PS(16:0/16:0), TMS_1_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | PS(16:0/16:0), TMS_1_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | PS(16:0/16:0), TMS_1_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | PS(16:0/16:0), TBDMS_1_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | PS(16:0/16:0), TBDMS_1_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | PS(16:0/16:0), TBDMS_1_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udj-0000393000-5d82d8d3c3cc3a8e1ef7 | 2016-09-19 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-0000016900-6bb9b6bacde6d8b4611b | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0000002900-eaf2a2217a8f79f1c16c | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0090033100-708416ca737a3b1fee06 | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000010900-db7bc8c2e3b9dad9960c | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udr-0001391600-51c373de4449f596bd5c | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0001391100-9a4a9ad709f158064e08 | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-14i6-0000099900-38c346454df0c2d55b3a | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0k96-0900099900-cf1ed9c46228bdf4f23f | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k96-0900099900-cf1ed9c46228bdf4f23f | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000000900-402c44be8653ebc0c284 | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0000001900-2b5ae18fbcabd46b8f69 | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0535-0166109600-e8d10538897e7b62801e | 2021-09-24 | View Spectrum |
|
|---|
| NMR | Not Available |
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Phospholipid Biosynthesis |    | 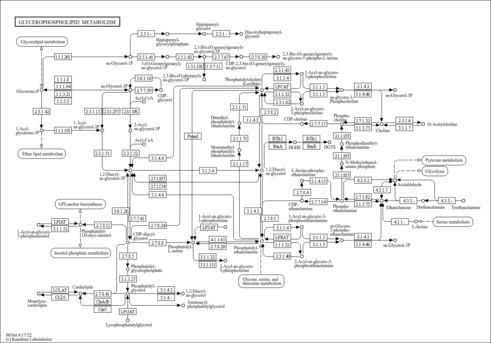 | | Phosphatidylcholine Biosynthesis PC(16:0/16:0) |  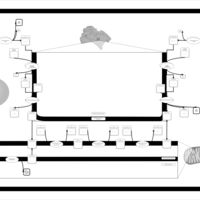 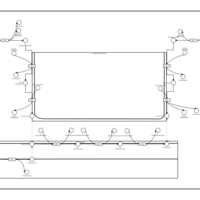 | Not Available | | Phosphatidylethanolamine Biosynthesis PE(16:0/16:0) | 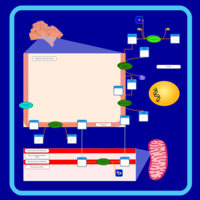 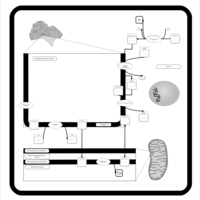  | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
|---|
| Transporters | |
| Probable phospholipid-transporting ATPase IA | ATP8A1 | 4p13 | Q9Y2Q0 | details |
|
|---|
| Metal Bindings | |
|---|
| Receptors | |
|---|
| Transcriptional Factors | |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0000614 |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB022144 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 7826040 |
|---|
| KEGG Compound ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 9547090 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 84523 |
|---|
| References |
|---|
| General References | Not Available |
|---|
