| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-04-27 16:55:21 UTC |
|---|
| Updated at | 2021-01-22 17:44:17 UTC |
|---|
| CannabisDB ID | CDB005775 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | Decanoic acid |
|---|
| Description | Decanoic acid, also known by capric acid has the molecular formula of C10H20O2. Decanoic acid is a white crystalline solid with a rancid odor and a melting point of 31.5°C. Soluble in most organic solvents and in dilute nitric acid. Decanoic acid is industrially used to make esters for perfumes and fruit flavors and as an intermediate for food-grade additives. Decanoic acid is a 10 carbon atoms long, straight-chain, saturated fatty acid. It has been described as an antibacterial agent, an anti-inflammatory agent, a human metabolite, a volatile oil component, a plant metabolite and an algal metabolite. It is the conjugate acid of a decanoate. Capric acid is found naturally in the coconut and palm kernel oils as well as the milk of various mammals. It is widespread in plant oils and as glycerides in seed oils. Decanoic acid is volatilized during the combustion of cannabis and can be found in cannabis smoke (Ref: Ref:DOI ). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Nonanecarboxylic acid | ChEBI | | 10:0 | ChEBI | | C10:0 | ChEBI | | Caprinic acid | ChEBI | | CH3-[CH2]8-COOH | ChEBI | | Decanoate | ChEBI | | Decoic acid | ChEBI | | Decylic acid | ChEBI | | Dekansaeure | ChEBI | | Kaprinsaeure | ChEBI | | N-Capric acid | ChEBI | | N-Decanoic acid | ChEBI | | N-Decoic acid | ChEBI | | N-Decylic acid | ChEBI | | 1-Nonanecarboxylate | Generator | | Caprinate | Generator | | Decanoic acid | Generator | | Decoate | Generator | | Decylate | Generator | | N-Caprate | Generator | | N-Decanoate | Generator | | N-Decoate | Generator | | N-Decylate | Generator | | Caprate | Generator | | Caprynate | HMDB | | Caprynic acid | HMDB | | Emery 659 | HMDB | | Lunac 10-95 | HMDB | | Lunac 10-98 | HMDB | | Prifac 2906 | HMDB | | Prifac 296 | HMDB | | Decanoic acid, sodium salt | HMDB | | Sodium caprate | HMDB | | Sodium decanoate | HMDB | | FA(10:0) | HMDB |
|
|---|
| Chemical Formula | C10H20O2 |
|---|
| Average Molecular Weight | 172.26 |
|---|
| Monoisotopic Molecular Weight | 172.1463 |
|---|
| IUPAC Name | decanoic acid |
|---|
| Traditional Name | capric acid |
|---|
| CAS Registry Number | 334-48-5 |
|---|
| SMILES | CCCCCCCCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H20O2/c1-2-3-4-5-6-7-8-9-10(11)12/h2-9H2,1H3,(H,11,12) |
|---|
| InChI Key | GHVNFZFCNZKVNT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as medium-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 4 and 12 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Medium-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain fatty acid
- Straight chain fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Physiological effect | Health effect: |
|---|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Biological role: Industrial application: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 31.9 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.062 mg/mL | Not Available | | logP | 4.09 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| EI-MS | Mass Spectrum (Electron Ionization) | splash10-074l-9100000000-bf788cb34c09c6af56bf | 2014-09-20 | View Spectrum | | GC-MS | Decanoic acid, 1 TMS, GC-MS Spectrum | splash10-017i-2920000000-7f6721f01b80a790d544 | Spectrum | | GC-MS | Decanoic acid, non-derivatized, GC-MS Spectrum | splash10-076u-9000000000-96a2e9e00e464db3c086 | Spectrum | | GC-MS | Decanoic acid, non-derivatized, GC-MS Spectrum | splash10-017i-2920000000-7f6721f01b80a790d544 | Spectrum | | GC-MS | Decanoic acid, non-derivatized, GC-MS Spectrum | splash10-016r-1910000000-1cc1026f6f325d994ab4 | Spectrum | | Predicted GC-MS | Decanoic acid, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-052g-9200000000-71a57b0e200e46fb66ed | Spectrum | | Predicted GC-MS | Decanoic acid, 1 TMS, Predicted GC-MS Spectrum - 70eV, Positive | splash10-00g0-9300000000-c615f39c0b907a00f67c | Spectrum | | Predicted GC-MS | Decanoic acid, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Decanoic acid, TBDMS_1_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00di-0900000000-0a7f944302bce161f7e5 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00b9-1900000000-b68efbceecf3433a9995 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-005a-9600000000-f2a54ed1a56ee9b7af77 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80B) , Positive | splash10-076u-9000000000-96a2e9e00e464db3c086 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-00di-0900000000-771e7907916bf05e6b10 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-00di-0900000000-f1e000384728ee06f802 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-00di-1900000000-42a901bb54546da030da | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-004i-9000000000-83e77de04461ded1c4bc | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-004i-9000000000-f3190b828218d04d3cc7 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - ESI-TOF 10V, Negative | splash10-014i-0000000910-e82208ed71b4a3735258 | 2017-08-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - ESI-TOF , Negative | splash10-00di-0900000000-6ec01ce9b8fdc68d94a9 | 2017-08-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - ESI-TOF 20V, Negative | splash10-00di-0900000000-6ec01ce9b8fdc68d94a9 | 2017-08-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - ESI-TOF 10V, Negative | splash10-00di-0900000000-8c6cdf0491f51ba6ef26 | 2017-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-856f34ef153b15cb3d1d | 2015-04-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0adi-4900000000-efb0bd73973bf0c317fd | 2015-04-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-d81148541fa575d32552 | 2015-04-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-99cd0519b210c46b4a4c | 2015-04-25 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fmi-1900000000-209285ec682ca47e1e5d | 2015-04-25 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9300000000-fb697080d761d0f48fe2 | 2015-04-25 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-856f34ef153b15cb3d1d | 2015-05-26 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0adi-4900000000-efb0bd73973bf0c317fd | 2015-05-26 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-d81148541fa575d32552 | 2015-05-26 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-99cd0519b210c46b4a4c | 2015-05-27 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fmi-1900000000-209285ec682ca47e1e5d | 2015-05-27 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9300000000-fb697080d761d0f48fe2 | 2015-05-27 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, CDCl3, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, CDCl3, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CDCl3, experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Beta Oxidation of Very Long Chain Fatty Acids |    | 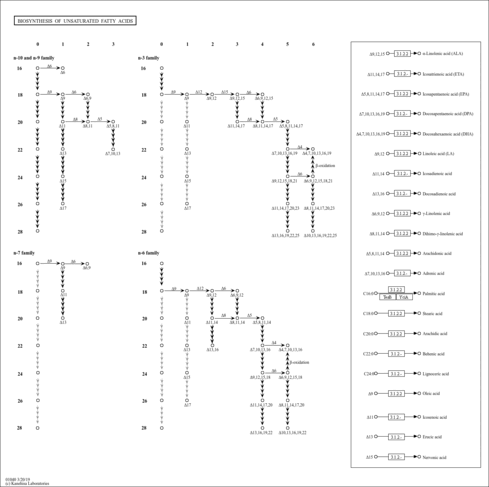 | | Fatty Acid Biosynthesis |    | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
| Peroxisomal carnitine O-octanoyltransferase | CROT | 7q21.1 | Q9UKG9 | details | | Carnitine O-palmitoyltransferase 2, mitochondrial | CPT2 | 1p32 | P23786 | details | | Mitochondrial carnitine/acylcarnitine carrier protein | SLC25A20 | 3p21.31 | O43772 | details |
|
|---|
| Transporters | |
| Mitochondrial carnitine/acylcarnitine carrier protein | SLC25A20 | 3p21.31 | O43772 | details | | Glycolipid transfer protein | GLTP | 12q24.11 | Q9NZD2 | details |
|
|---|
| Metal Bindings | Not Available |
|---|
| Receptors | Not Available |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0000511 |
|---|
| DrugBank ID | DB03600 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB012027 |
|---|
| KNApSAcK ID | C00001213 |
|---|
| Chemspider ID | 2863 |
|---|
| KEGG Compound ID | C01571 |
|---|
| BioCyc ID | CPD-3617 |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Decanoic acid |
|---|
| METLIN ID | 336 |
|---|
| PubChem Compound | 2969 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 30813 |
|---|
| References |
|---|
| General References | Not Available |
|---|
