| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-04-17 18:46:27 UTC |
|---|
| Updated at | 2020-11-18 16:38:55 UTC |
|---|
| CannabisDB ID | CDB004881 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | D-Sedoheptulose 7-phosphate |
|---|
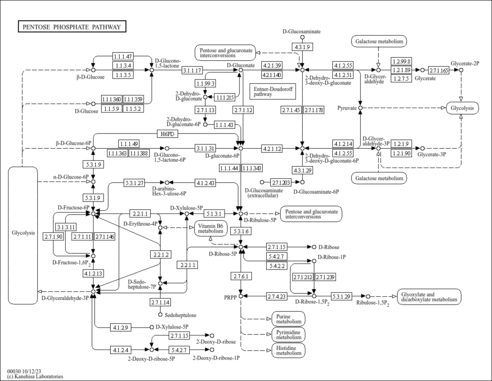
| Description | D-Sedoheptulose 7-phosphate belongs to the class of organic compounds known as hexose phosphates. These are carbohydrate derivatives containing a hexose substituted by one or more phosphate groups. D-Sedoheptulose 7-phosphate is an extremely weak basic (essentially neutral) compound (based on its pKa). Within humans, D-sedoheptulose 7-phosphate participates in a number of enzymatic reactions. In particular, D-glyceraldehyde 3-phosphate and D-sedoheptulose 7-phosphate can be converted into D-ribose 5-phosphate and xylulose 5-phosphate; which is mediated by the enzyme transketolase. In addition, D-glyceraldehyde 3-phosphate and D-sedoheptulose 7-phosphate can be biosynthesized from D-erythrose 4-phosphate and fructose 6-phosphate through its interaction with the enzyme transaldolase. In humans, D-sedoheptulose 7-phosphate is involved in the metabolic disorder called the glucose-6-phosphate dehydrogenase deficiency pathway. D-Sedoheptulose 7-phosphate, with regard to humans, has been found to be associated with several diseases such as frontotemporal dementia, lewy body disease, and alzheimer's disease; D-sedoheptulose 7-phosphate has also been linked to the inborn metabolic disorder transaldolase deficiency. A ketoheptose phosphate consisting of alpha-D-sedoheptulopyranose having a phosphate group at the 7-position. D-Sedoheptulose 7-phosphate is expected to be in Cannabis as all living plants are known to produce and metabolize it. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| alpha-D-Altro-hept-2-ulopyranose 7-hydrogen phosphate | ChEBI | | alpha-D-Sedoheptulose 7-phosphate | ChEBI | | a-D-Altro-hept-2-ulopyranose 7-hydrogen phosphate | Generator | | a-D-Altro-hept-2-ulopyranose 7-hydrogen phosphoric acid | Generator | | alpha-D-Altro-hept-2-ulopyranose 7-hydrogen phosphoric acid | Generator | | Α-D-altro-hept-2-ulopyranose 7-hydrogen phosphate | Generator | | Α-D-altro-hept-2-ulopyranose 7-hydrogen phosphoric acid | Generator | | a-D-Sedoheptulose 7-phosphate | Generator | | a-D-Sedoheptulose 7-phosphoric acid | Generator | | alpha-D-Sedoheptulose 7-phosphoric acid | Generator | | Α-D-sedoheptulose 7-phosphate | Generator | | Α-D-sedoheptulose 7-phosphoric acid | Generator | | D-Sedoheptulose 7-phosphoric acid | Generator | | a-D-Sedoheptulopyranose 7-phosphate | HMDB | | a-D-Sedoheptulopyranose 7-phosphoric acid | HMDB | | alpha-D-Sedoheptulopyranose 7-phosphoric acid | HMDB | | Α-D-sedoheptulopyranose 7-phosphate | HMDB | | Α-D-sedoheptulopyranose 7-phosphoric acid | HMDB | | D-Altro-heptulose 7-phosphate | HMDB | | Sedoheptulose 7-phosphate | HMDB | | Altro-heptulose 7-phosphate | HMDB | | D-Sedoheptulose 7-phosphate | HMDB |
|
|---|
| Chemical Formula | C7H15O10P |
|---|
| Average Molecular Weight | 290.16 |
|---|
| Monoisotopic Molecular Weight | 290.0403 |
|---|
| IUPAC Name | {[(2R,3S,4R,5S,6S)-3,4,5,6-tetrahydroxy-6-(hydroxymethyl)oxan-2-yl]methoxy}phosphonic acid |
|---|
| Traditional Name | [(2R,3S,4R,5S,6S)-3,4,5,6-tetrahydroxy-6-(hydroxymethyl)oxan-2-yl]methoxyphosphonic acid |
|---|
| CAS Registry Number | 89927-08-2 |
|---|
| SMILES | OC[C@]1(O)O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C7H15O10P/c8-2-7(12)6(11)5(10)4(9)3(17-7)1-16-18(13,14)15/h3-6,8-12H,1-2H2,(H2,13,14,15)/t3-,4-,5-,6+,7+/m1/s1 |
|---|
| InChI Key | CBIDVWSRUUODHL-OVHBTUCOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as hexose phosphates. These are carbohydrate derivatives containing a hexose substituted by one or more phosphate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Hexose phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose phosphate
- C-glycosyl compound
- Glycosyl compound
- Monosaccharide phosphate
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Alkyl phosphate
- Hemiacetal
- Secondary alcohol
- Organoheterocyclic compound
- Oxacycle
- Polyol
- Organic oxide
- Alcohol
- Primary alcohol
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | logP | Not Available | Not Available |
|
|---|
| Predicted Properties | [] |
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0290000000-5d8d1cd1634c1742e9e7 | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-4950000000-1ab2e06e4ad5b21ccedd | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-9100000000-a0c646f88a2206fcdd12 | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002b-9010000000-16bc2a0cc5bddeca6aaf | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9010000000-d1c071e2fcd61cb5ded3 | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-bc05f33d90e2bf18235c | 2021-09-22 | View Spectrum |
|
|---|