| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-04-17 18:36:37 UTC |
|---|
| Updated at | 2020-12-07 19:10:55 UTC |
|---|
| CannabisDB ID | CDB004786 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | Biotin |
|---|
| Description | Biotin, also known as D-biotin or coenzyme R, belongs to the class of organic compounds known as biotin and derivatives. These are organic compounds containing a ureido (tetrahydroimidizalone) ring fused with a tetrahydrothiophene ring. Biotin is a drug which is used for nutritional supplementation, also for treating dietary shortage or imbalance. Biotin is an extremely weak basic (essentially neutral) compound (based on its pKa). An organic heterobicyclic compound that consists of 2-oxohexahydro-1H-thienoimidazole having a valeric acid substituent attached to the tetrahydrothiophene ring. Biotin exists in all living species, ranging from bacteria to humans. In humans, biotin is involved in the metabolic disorder called the multiple carboxylase deficiency, neonatal or early onset form pathway. Biotin is a potentially toxic compound. Biotin is expected to be in Cannabis as all living plants are known to produce and metabolize it. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-cis-Hexahydro-2-oxo-1H-thieno[3,4]imidazole-4-valeric acid | ChEBI | | (3AS,4S,6ar)-hexahydro-2-oxo-1H-thieno[3,4-D]imidazole-4-valeric acid | ChEBI | | 5-(2-Oxohexahydro-1H-thieno[3,4-D]imidazol-4-yl)pentanoic acid | ChEBI | | Biotina | ChEBI | | Biotine | ChEBI | | Biotinum | ChEBI | | cis-(+)-Tetrahydro-2-oxothieno[3,4]imidazoline-4-valeric acid | ChEBI | | cis-Hexahydro-2-oxo-1H-thieno(3,4)imidazole-4-valeric acid | ChEBI | | cis-Tetrahydro-2-oxothieno(3,4-D)imidazoline-4-valeric acid | ChEBI | | Coenzyme R | ChEBI | | D-(+)-Biotin | ChEBI | | D-Biotin | ChEBI | | Vitamin b7 | ChEBI | | Vitamin H | ChEBI | | Bioepiderm | Kegg | | (+)-cis-Hexahydro-2-oxo-1H-thieno[3,4]imidazole-4-valerate | Generator | | (3AS,4S,6ar)-hexahydro-2-oxo-1H-thieno[3,4-D]imidazole-4-valerate | Generator | | 5-(2-Oxohexahydro-1H-thieno[3,4-D]imidazol-4-yl)pentanoate | Generator | | cis-(+)-Tetrahydro-2-oxothieno[3,4]imidazoline-4-valerate | Generator | | cis-Hexahydro-2-oxo-1H-thieno(3,4)imidazole-4-valerate | Generator | | cis-Tetrahydro-2-oxothieno(3,4-D)imidazoline-4-valerate | Generator | | (+)-Biotin | HMDB | | -(+)-Biotin | HMDB | | 1SWK | HMDB | | 1SWN | HMDB | | 1SWR | HMDB | | Biodermatin | HMDB | | Bios H | HMDB | | Bios II | HMDB | | D(+)-Biotin | HMDB | | D-Biotin factor S | HMDB | | delta-(+)-Biotin | HMDB | | delta-Biotin | HMDB | | delta-Biotin factor S | HMDB | | Factor S | HMDB | | Factor S (vitamin) | HMDB | | Hexahydro-2-oxo-1H-thieno(3,4-D)imidazole-4-pentanoate | HMDB | | Hexahydro-2-oxo-1H-thieno(3,4-D)imidazole-4-pentanoic acid | HMDB | | Hexahydro-2-oxo-[3as-(3aa,4b,6aa)]-1H-thieno[3,4-D]imidazole-4-pentanoate | HMDB | | Hexahydro-2-oxo-[3as-(3aa,4b,6aa)]-1H-thieno[3,4-D]imidazole-4-pentanoic acid | HMDB | | Hexahydro-2-oxo-[3as-(3alpha,4beta,6alpha)]-1H-thieno[3,4-D]imidazole-4-pentanoate | HMDB | | Hexahydro-2-oxo-[3as-(3alpha,4beta,6alpha)]-1H-thieno[3,4-D]imidazole-4-pentanoic acid | HMDB | | Lutavit H2 | HMDB | | Meribin | HMDB | | Rovimix H 2 | HMDB | | Vitamin-H | HMDB | | Biotin hermes brand | HMDB | | Biotin roche brand | HMDB | | Biotin simons brand | HMDB | | Biotin strathmann brand | HMDB | | Biotin ziethen brand | HMDB | | Biotin ratiopharm | HMDB | | Roche brand OF biotin | HMDB | | Roche, biotine | HMDB | | E+b pharma brand OF biotin | HMDB | | Medobiotin | HMDB | | Biokur | HMDB | | Biotinratiopharm | HMDB | | Gelfert, biotin | HMDB | | Hermes brand OF biotin | HMDB | | Hermes, biotin | HMDB | | Medopharm brand OF biotin | HMDB | | Ratiopharm brand OF biotin | HMDB | | Biocur brand OF biotin | HMDB | | Biotin dermapharm brand | HMDB | | Biotine roche | HMDB | | Deacura | HMDB | | Dermapharm brand OF biotin | HMDB | | Gabunat | HMDB | | Medebiotin | HMDB | | Rombellin | HMDB | | Ziethen brand OF biotin | HMDB | | Biotin biocur brand | HMDB | | Biotin gelfert | HMDB | | Biotin hermes | HMDB | | Biotin medopharm brand | HMDB | | Biotin ratiopharm brand | HMDB | | Biotin-ratiopharm | HMDB | | H, Vitamin | HMDB | | Medea brand OF biotin sodium salt | HMDB | | Simons brand OF biotin | HMDB | | Strathmann brand OF biotin | HMDB |
|
|---|
| Chemical Formula | C10H16N2O3S |
|---|
| Average Molecular Weight | 244.31 |
|---|
| Monoisotopic Molecular Weight | 244.0882 |
|---|
| IUPAC Name | 5-[(3aS,4S,6aR)-2-oxo-hexahydro-1H-thieno[3,4-d]imidazol-4-yl]pentanoic acid |
|---|
| Traditional Name | 5-[(3aS,4S,6aR)-2-oxo-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoic acid |
|---|
| CAS Registry Number | 58-85-5 |
|---|
| SMILES | [H][C@]12CS[C@@H](CCCCC(O)=O)[C@@]1([H])NC(=O)N2 |
|---|
| InChI Identifier | InChI=1S/C10H16N2O3S/c13-8(14)4-2-1-3-7-9-6(5-16-7)11-10(15)12-9/h6-7,9H,1-5H2,(H,13,14)(H2,11,12,15)/t6-,7-,9-/m0/s1 |
|---|
| InChI Key | YBJHBAHKTGYVGT-ZKWXMUAHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as biotin and derivatives. These are organic compounds containing a ureido (tetrahydroimidizalone) ring fused with a tetrahydrothiophene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Biotin and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Biotin and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biotin
- Imidazolyl carboxylic acid derivative
- Medium-chain fatty acid
- Heterocyclic fatty acid
- Thia fatty acid
- Fatty acid
- Fatty acyl
- Thiolane
- 2-imidazoline
- Isourea
- Azacycle
- Dialkylthioether
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Carboxylic acid derivative
- Thioether
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Organonitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Industrial application: Biological role: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 232 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.22 mg/mL at 25 °C | Not Available | | logP | Not Available | Not Available |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| EI-MS | Mass Spectrum (Electron Ionization) | splash10-01pt-9500000000-a1e1ec56cf32236ac6b1 | 2018-05-25 | View Spectrum | | GC-MS | Biotin, non-derivatized, GC-MS Spectrum | splash10-0f76-1960000000-b21ddd69490cac3254f8 | Spectrum | | GC-MS | Biotin, 3 TMS, GC-MS Spectrum | splash10-0f76-3960000000-a8a94e2de123f66979d8 | Spectrum | | GC-MS | Biotin, non-derivatized, GC-MS Spectrum | splash10-0f76-1960000000-b21ddd69490cac3254f8 | Spectrum | | GC-MS | Biotin, non-derivatized, GC-MS Spectrum | splash10-0f76-3960000000-a8a94e2de123f66979d8 | Spectrum | | Predicted GC-MS | Biotin, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-0006-6910000000-11bfe0a5a77f7dfaa8c5 | Spectrum | | Predicted GC-MS | Biotin, 1 TMS, Predicted GC-MS Spectrum - 70eV, Positive | splash10-00dl-9680000000-eb01d8147a82f7982b54 | Spectrum | | Predicted GC-MS | Biotin, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Biotin, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Biotin, TMS_1_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Biotin, TMS_1_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Biotin, TBDMS_1_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Biotin, TBDMS_1_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Biotin, TBDMS_1_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0006-0090000000-6d956bb533d353d449c9 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0006-0190000000-01f67d1bdf8c742e48c8 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0fxx-3920000000-f0b9613cbd9371e4be92 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0006-9400000000-107f2a44f521c2513578 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0006-9000000000-1a3f65d909dc40055e87 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-004i-0090000000-c928e8d0a18f3f848262 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-066s-1920000000-5f795e0b7f1d7cf986e5 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-0ar1-1920000000-c298be862857cb3bbc7f | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-000t-0900000000-cdc4a4c359ff765fd32d | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-05xs-1920000000-3be430b63e9c748c681a | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0006-0090000000-bcffb0dcf77e8fd727a4 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0f6x-0390000000-c209523d36e9a7681f44 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-0090000000-c0f81ee86772310db415 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-0190000000-016eb89528e564c74720 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0fxx-3920000000-f0b9613cbd9371e4be92 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-9400000000-107f2a44f521c2513578 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-9000000000-d08e1b3709844e1e91b0 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0006-0190000000-21d0f2512788276bbb89 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-0udj-0690000000-dc372934024e58bdcc60 | 2017-09-14 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0290000000-43932f104dea28cbdfb8 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-1960000000-d3673c6bc624b97f35be | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9600000000-00a4e152d89024a9b423 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1390000000-3f4b512cfa57b894ff94 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6y-7890000000-ea1e647fc5b31e42af0b | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-e139c928dcc46b225bdb | 2016-09-12 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, D2O, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, D2O, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | | Spectrum | | 2D NMR | [1H, 1H]-TOCSY. Unexported temporarily by An Chi on Oct 15, 2021 until json or nmrML file is generated. 2D NMR Spectrum (experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Biotin Metabolism |    | 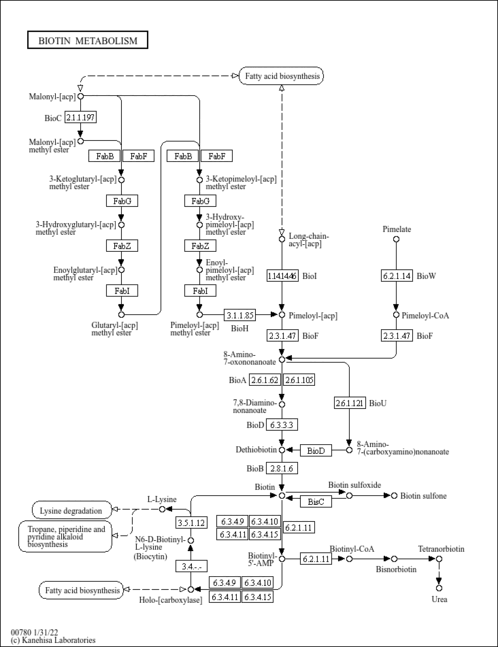 | | Alanine Metabolism |    |  | | Primary Hyperoxaluria Type I |    | Not Available | | Pyruvate Carboxylase Deficiency |    | Not Available | | Lactic Acidemia |    | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
|---|
| Transporters | |
| Sodium-dependent multivitamin transporter | SLC5A6 | 2p23 | Q9Y289 | details |
|
|---|
| Metal Bindings | |
|---|
| Receptors | Not Available |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0000030 |
|---|
| DrugBank ID | DB00121 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB014510 |
|---|
| KNApSAcK ID | C00000756 |
|---|
| Chemspider ID | 149962 |
|---|
| KEGG Compound ID | C00120 |
|---|
| BioCyc ID | BIOTIN |
|---|
| BiGG ID | 33931 |
|---|
| Wikipedia Link | Biotin |
|---|
| METLIN ID | 243 |
|---|
| PubChem Compound | 171548 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 15956 |
|---|
| References |
|---|
| General References | Not Available |
|---|

