| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-04-17 18:48:48 UTC |
|---|
| Updated at | 2020-11-18 16:38:58 UTC |
|---|
| CannabisDB ID | CDB004903 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | 4-Fumarylacetoacetic acid |
|---|
| Description | 4-Fumarylacetoacetic acid, also known as fumarylacetoacetate, belongs to the class of organic compounds known as medium-chain keto acids and derivatives. These are keto acids with a 6 to 12 carbon atoms long side chain. 4-Fumarylacetoacetic acid is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. Within humans, 4-fumarylacetoacetic acid participates in a number of enzymatic reactions. In particular, 4-fumarylacetoacetic acid can be biosynthesized from maleylacetoacetic acid through its interaction with the enzyme maleylacetoacetate isomerase. In addition, 4-fumarylacetoacetic acid can be converted into acetoacetic acid and fumaric acid; which is mediated by the enzyme fumarylacetoacetase. In humans, 4-fumarylacetoacetic acid is involved in the metabolic disorder called tyrosinemia type 3 (tyro3). Outside of the human body, 4-Fumarylacetoacetic acid has been detected, but not quantified in, several different foods, such as walnuts, lupines, narrowleaf cattails, wild carrots, and japanese walnuts. This could make 4-fumarylacetoacetic acid a potential biomarker for the consumption of these foods. 4-Fumarylacetoacetic acid is expected to be in Cannabis as all living plants are known to produce and metabolize it. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Fumarylacetoacetone | ChEBI | | Fumarylacetoacetate | Kegg | | Fumarylacetoacetic acid | Generator | | 4-Fumarylacetoacetate | Generator | | 4-Fumaryl-acetoacetate | HMDB | | (2E)-4,6-Dioxo-2-octenedioic acid | HMDB | | 4-Fumarylacetoacetic acid | HMDB |
|
|---|
| Chemical Formula | C8H8O6 |
|---|
| Average Molecular Weight | 200.15 |
|---|
| Monoisotopic Molecular Weight | 200.0321 |
|---|
| IUPAC Name | (2E)-4,6-dioxooct-2-enedioic acid |
|---|
| Traditional Name | 4-fumarylacetoacetic acid |
|---|
| CAS Registry Number | 28613-33-4 |
|---|
| SMILES | OC(=O)CC(=O)CC(=O)\C=C\C(O)=O |
|---|
| InChI Identifier | InChI=1S/C8H8O6/c9-5(1-2-7(11)12)3-6(10)4-8(13)14/h1-2H,3-4H2,(H,11,12)(H,13,14)/b2-1+ |
|---|
| InChI Key | GACSIVHAIFQKTC-OWOJBTEDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as medium-chain keto acids and derivatives. These are keto acids with a 6 to 12 carbon atoms long side chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Keto acids and derivatives |
|---|
| Sub Class | Medium-chain keto acids and derivatives |
|---|
| Direct Parent | Medium-chain keto acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain keto acid
- Beta-keto acid
- 1,3-diketone
- Beta-hydroxy ketone
- Dicarboxylic acid or derivatives
- Unsaturated fatty acid
- 1,3-dicarbonyl compound
- Fatty acyl
- Enone
- Acryloyl-group
- Alpha,beta-unsaturated ketone
- Ketone
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | logP | Not Available | Not Available |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | 4-Fumarylacetoacetic acid, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-0007-9300000000-7b2ae90eb353073cb652 | Spectrum | | Predicted GC-MS | 4-Fumarylacetoacetic acid, 2 TMS, Predicted GC-MS Spectrum - 70eV, Positive | splash10-05bu-7092000000-254fb0c9b4683fd48ef1 | Spectrum | | Predicted GC-MS | 4-Fumarylacetoacetic acid, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | 4-Fumarylacetoacetic acid, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fsi-1920000000-f36f976fb4a0271e936b | 2016-08-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-6900000000-692cbcc9f99aac913e42 | 2016-08-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00rj-9200000000-8e21be4180492b988f54 | 2016-08-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4j-1900000000-ea634f49698a94be6311 | 2016-08-03 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-5900000000-4d5261faac7c9dc29c36 | 2016-08-03 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9500000000-17b0a81ad882f626ac2a | 2016-08-03 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0bt9-3900000000-0353447aa417ab1cef29 | 2021-09-23 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9600000000-fabbd099eded8f7879a9 | 2021-09-23 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9000000000-e4bd7674e02d56b3573f | 2021-09-23 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001r-4900000000-80aa92977bd40f96e740 | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-9100000000-7d688d7f7d7a804690a3 | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-c0d2eba6487ff950c63d | 2021-09-24 | View Spectrum |
|
|---|
| NMR | Not Available |
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Tyrosine Metabolism | 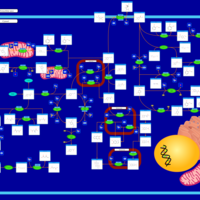 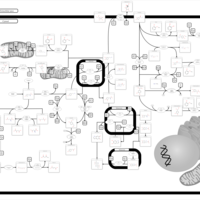 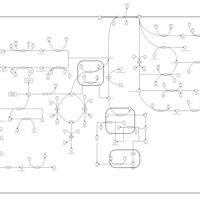 |  | | Phenylalanine and Tyrosine Metabolism | 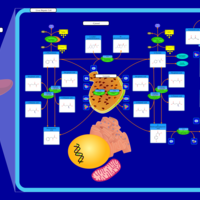 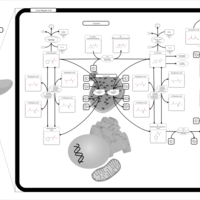 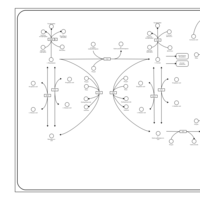 | 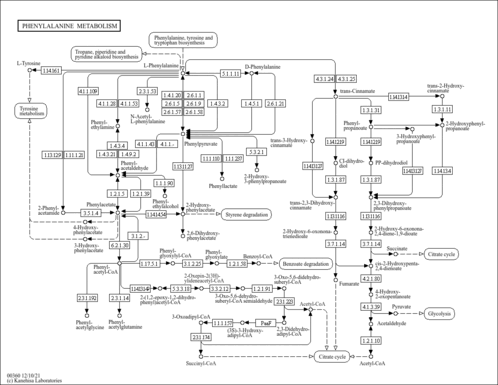 | | Phenylketonuria |  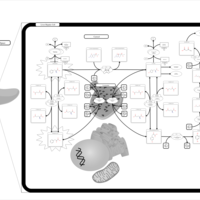 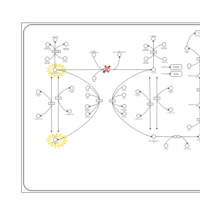 | Not Available | | Tyrosinemia Type 2 (or Richner-Hanhart syndrome) | 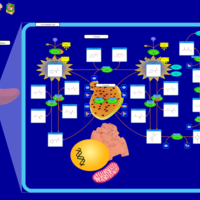 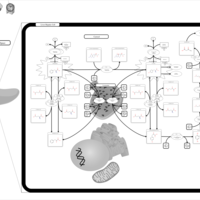 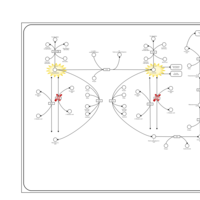 | Not Available | | Tyrosinemia Type 3 (TYRO3) | 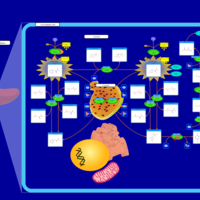 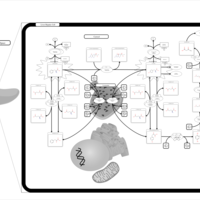 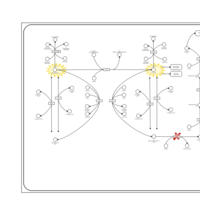 | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
|---|
| Transporters | Not Available |
|---|
| Metal Bindings | |
|---|
| Receptors | Not Available |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0001268 |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB022817 |
|---|
| KNApSAcK ID | C00007599 |
|---|
| Chemspider ID | 4444081 |
|---|
| KEGG Compound ID | C01061 |
|---|
| BioCyc ID | 4-FUMARYL-ACETOACETATE |
|---|
| BiGG ID | 1485275 |
|---|
| Wikipedia Link | Fumarylacetoacetic_acid |
|---|
| METLIN ID | 6123 |
|---|
| PubChem Compound | 5280398 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 30907 |
|---|
| References |
|---|
| General References | Not Available |
|---|

