| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-03-18 23:22:21 UTC |
|---|
| Updated at | 2022-12-13 23:36:26 UTC |
|---|
| CannabisDB ID | CDB006168 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | Tyramine |
|---|
| Description | Tyramine is a monoamine compound derived from the amino acid tyrosine. It is a very strong basic compound (based on its pKa). Tyramine is metabolized by the enzyme monoamine oxidase. In foods, it is often produced by the decarboxylation of tyrosine during fermentation or decay. Foods containing considerable amounts of tyramine include fish (herring), chocolate, alcoholic beverages, cheese, yogurt, soy sauce, sauerkraut, and processed meat. Tyramine in the blood or urine is often considered as a biomarker for the consumption of cheese. A large dietary intake of tyramine can cause an increase in systolic blood pressure of 30 mmHg or more and induce migraine headaches (PMID: 27424325 ). The tyramine connection to hypertensive episodes was discovered by a British pharmacist whose wife was taking an monoamine oxidase inhibitors (MAOI). He noticed that every time they had a meal with cheese, she would get a severe headache (PMID: 19742203 ). Tyramine acts as a neurotransmitter via a G protein-coupled receptor called TA1, which has a high affinity for tyramine. The TA1 receptor is found in the brain as well as peripheral tissues including the kidney. As an indirect sympathomimetic compound, tyramine can also serve as a substrate for adrenergic uptake systems and monoamine oxidase, so it prolongs the actions of adrenergic transmitters. Tyramine also provokes transmitter release from adrenergic terminals. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(p-Hydroxyphenyl)ethylamine | ChEBI | | 4-Hydroxy-beta-phenylethylamine | ChEBI | | 4-Hydroxyphenethylamine | ChEBI | | 4-Hydroxyphenylethylamine | ChEBI | | beta-(4-Hydroxyphenyl)ethylamine | ChEBI | | p-(2-Aminoethyl)phenol | ChEBI | | p-Hydroxyphenethylamine | ChEBI | | p-Hydroxyphenylethylamine | ChEBI | | p-Tyramine | ChEBI | | Tyramin | ChEBI | | 4-Hydroxy-b-phenylethylamine | Generator | | 4-Hydroxy-β-phenylethylamine | Generator | | b-(4-Hydroxyphenyl)ethylamine | Generator | | Β-(4-hydroxyphenyl)ethylamine | Generator | | 2-(4'-Hydroxyphenyl)ethylamine | HMDB | | 2-(4-Hydroxyphenyl)ethylamine | HMDB | | 4-(2-Aminoethyl)-phenol | HMDB | | 4-(2-Aminoethyl)-phenol(thyramin) | HMDB | | 4-(2-Aminoethyl)phenol | HMDB | | 4-Hydroxy-benzeneethanamine | HMDB | | a-(4-Hydroxyphenyl)-b-aminoethane | HMDB | | alpha-(4-Hydroxyphenyl)-beta-aminoethane | HMDB | | p-(2-Aminoethyl)-phenol | HMDB | | p-beta-Aminoethylphenol | HMDB | | p-Hydroxy-b-phenethylamine | HMDB | | p-Hydroxy-b-phenylethylamine | HMDB | | p-Hydroxy-beta-phenethylamine | HMDB | | p-Hydroxy-beta-phenylethylamine | HMDB | | Systogene | HMDB | | Tenosin-wirkstoff | HMDB | | Tocosine | HMDB | | Tyramine base | HMDB | | Tyrosamine | HMDB | | Uteramine | HMDB | | Para-tyramine | HMDB | | 4 Hydroxyphenethylamine | HMDB | | 2-(4-Hydroxyphenyl)ethanamine | HMDB | | 2-(4’-hydroxyphenyl)ethylamine | HMDB | | p-Hydroxy-β-phenylethylamine | HMDB | | p-Β-aminoethylphenol | HMDB | | Α-(4-hydroxyphenyl)-β-aminoethane | HMDB |
|
|---|
| Chemical Formula | C8H11NO |
|---|
| Average Molecular Weight | 137.18 |
|---|
| Monoisotopic Molecular Weight | 137.0841 |
|---|
| IUPAC Name | 4-(2-aminoethyl)phenol |
|---|
| Traditional Name | tyramine |
|---|
| CAS Registry Number | 51-67-2 |
|---|
| SMILES | NCCC1=CC=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 |
|---|
| InChI Key | DZGWFCGJZKJUFP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as phenethylamines. Phenethylamines are compounds containing a phenethylamine moiety, which consists of a phenyl group substituted at the second position by an ethan-1-amine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenethylamines |
|---|
| Direct Parent | Phenethylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenethylamine
- 2-arylethylamine
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Phenol
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Physiological effect | Health effect: |
|---|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Biological role: Industrial application: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 164 - 165 °C | Not Available | | Boiling Point | 206 °C at 25 mmHg | Wikipedia | | Water Solubility | 10.4 mg/mL at 15 °C | Not Available | | logP | Not Available | Not Available |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| EI-MS | Mass Spectrum (Electron Ionization) | splash10-053r-9500000000-6deb2b5d214f768ca448 | 2014-09-20 | View Spectrum | | GC-MS | Tyramine, 3 TMS, GC-MS Spectrum | splash10-00di-2900000000-273503edc6a220e152b6 | Spectrum | | GC-MS | Tyramine, non-derivatized, GC-MS Spectrum | splash10-00di-2900000000-80980d19fa83d4b86f91 | Spectrum | | GC-MS | Tyramine, 3 TMS, GC-MS Spectrum | splash10-00di-9700000000-78d2ae48da1eee18358e | Spectrum | | GC-MS | Tyramine, non-derivatized, GC-MS Spectrum | splash10-001i-9200000000-50c21473a7d48011358c | Spectrum | | GC-MS | Tyramine, non-derivatized, GC-MS Spectrum | splash10-00di-1900000000-9b1000a8978b57919c35 | Spectrum | | GC-MS | Tyramine, non-derivatized, GC-MS Spectrum | splash10-00di-2900000000-273503edc6a220e152b6 | Spectrum | | GC-MS | Tyramine, non-derivatized, GC-MS Spectrum | splash10-00di-2900000000-80980d19fa83d4b86f91 | Spectrum | | GC-MS | Tyramine, non-derivatized, GC-MS Spectrum | splash10-00di-9700000000-78d2ae48da1eee18358e | Spectrum | | GC-MS | Tyramine, non-derivatized, GC-MS Spectrum | splash10-00di-2900000000-69892c95ab2778a208b3 | Spectrum | | Predicted GC-MS | Tyramine, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-001i-9600000000-1d5276a762318a00832d | Spectrum | | Predicted GC-MS | Tyramine, 1 TMS, Predicted GC-MS Spectrum - 70eV, Positive | splash10-0fk9-7900000000-8f3b164f36bc5a4df4d7 | Spectrum | | Predicted GC-MS | Tyramine, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Tyramine, TMS_1_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Tyramine, TBDMS_1_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Tyramine, TBDMS_1_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| MS/MS | LC-MS/MS Spectrum - , negative | splash10-000i-0900000000-066947a256844eb6cbdc | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-000i-0900000000-4b0f190d7304f2cec82d | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-2feedb2abf4fe5413452 | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-052r-0900000000-e67940e0a4f0f1b565c4 | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-00lr-9400000000-47a2e802a22167ed8a63 | 2021-09-20 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00di-0900000000-3229081a448a277191a2 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-004l-9300000000-e2fc12d41e4c40a9a296 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-004i-9000000000-92f0b12d0a64443cede8 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - EI-B (Unknown) , Positive | splash10-001i-9200000000-50c21473a7d48011358c | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000i-0900000000-d7e9a8705227b4504c5c | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0006-9000000000-3f02b334907c40a8315c | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-00di-0900000000-e98b3afc6fad78d45d38 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0900000000-54239e66d89de3096ca8 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0079-0902000000-f09759fc06ef461f3aa5 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0006-9000000000-9ffe6ee0ce68182216f7 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-00di-0900000000-020a5decfb44fc4f97df | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-004i-0930000000-32ccc375e75dbab3195c | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-00di-0900000000-51c6cc08017f1672b791 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-00di-1900000000-55c25dd64462c06c5316 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-0f96-9500000000-83a70aec6da00b51a6df | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-004i-9100000000-5f044e831c0d2db255fa | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-004i-9000000000-99edae3913507430bf9f | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-00di-4900000000-8944003750ac547c2024 | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00di-0900000000-00e85becc8cfc59bdb10 | 2017-09-14 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00di-0900000000-58e8539844dcdbc977cc | 2017-09-14 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, DMSO-d6, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | | Spectrum | | 2D NMR | [1H, 1H]-TOCSY. Unexported temporarily by An Chi on Oct 15, 2021 until json or nmrML file is generated. 2D NMR Spectrum (experimental) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Tyrosine Metabolism | 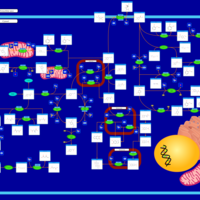 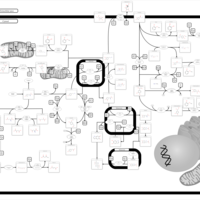 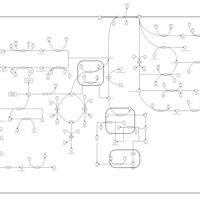 |  | | Alkaptonuria | 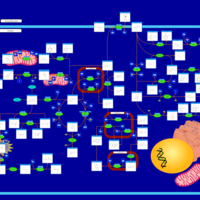 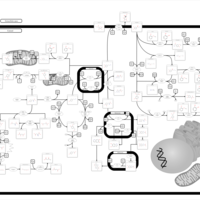  | Not Available | | Hawkinsinuria | 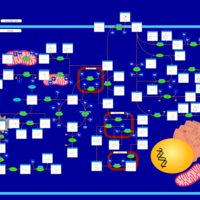   | Not Available | | Tyrosinemia Type I | 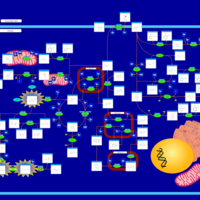 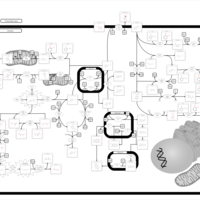 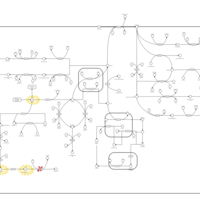 | Not Available | | Tyrosinemia, transient, of the newborn | 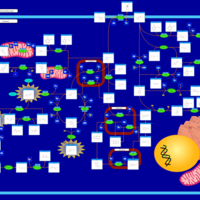 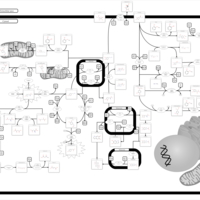 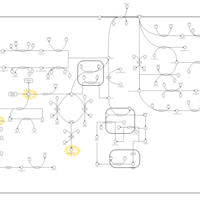 | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
|---|
| Transporters | Not Available |
|---|
| Metal Bindings | Not Available |
|---|
| Receptors | |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| |
| Alien Dawg | Detected and Quantified | 0.0124 mg/g dry wt | | details | | Gabriola | Detected and Quantified | 0.00362 mg/g dry wt | | details | | Island Honey | Detected and Quantified | 0.00728 mg/g dry wt | | details | | Quadra | Detected and Quantified | 0.00881 mg/g dry wt | | details | | Sensi Star | Detected and Quantified | 0.00786 mg/g dry wt | | details | | Tangerine Dream | Detected and Quantified | 0.0407 mg/g dry wt | | details |
|
|---|
| External Links |
|---|
| HMDB ID | HMDB0000306 |
|---|
| DrugBank ID | DB08841 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB000433 |
|---|
| KNApSAcK ID | C00001435 |
|---|
| Chemspider ID | 5408 |
|---|
| KEGG Compound ID | C00483 |
|---|
| BioCyc ID | TYRAMINE |
|---|
| BiGG ID | 35110 |
|---|
| Wikipedia Link | Tyramine |
|---|
| METLIN ID | 60 |
|---|
| PubChem Compound | 5610 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 15760 |
|---|
| References |
|---|
| General References | - Khan MZ, Nawaz W: The emerging roles of human trace amines and human trace amine-associated receptors (hTAARs) in central nervous system. Biomed Pharmacother. 2016 Oct;83:439-449. doi: 10.1016/j.biopha.2016.07.002. Epub 2016 Jul 15. [PubMed:27424325 ]
- Sathyanarayana Rao TS, Yeragani VK: Hypertensive crisis and cheese. Indian J Psychiatry. 2009 Jan;51(1):65-6. doi: 10.4103/0019-5545.44910. [PubMed:19742203 ]
|
|---|
