| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-03-18 23:22:11 UTC |
|---|
| Updated at | 2020-12-07 19:06:57 UTC |
|---|
| CannabisDB ID | CDB006163 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | Galactitol |
|---|
| Description | Galactitol or dulcitol is a sugar alcohol that is a metabolic breakdown product of galactose. Galactitol is an extremely weak basic (essentially neutral) compound (based on its pKa). Galactose is derived from lactose in food (such as dairy products). When lactose is broken down by the enzyme lactase it produces glucose and galactose. It is this galactose that is broken down to galactitol via a reaction catalyzed by aldose reductase. Galactitol has a slightly sweet taste. When present in sufficiently high levels, galactitol can act as a metabotoxin, a neurotoxin, and a hepatotoxin. A neurotoxin is a compound that disrupts or attacks neural cells and neural tissue. A hepatotoxin as a compound that disrupts or attacks liver tissue or liver cells. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. Chronically high levels of galactitol are associated with at least two inborn errors of metabolism, including galactosemia and galactosemia type II. Galactosemia is a rare genetic metabolic disorder that affects an individual's ability to metabolize the sugar galactose properly. Excess lactose consumption in individuals with galactose intolerance or galactosemia activates aldose reductase to produce galactitol, thus depleting NADPH and leading to lowered glutathione reductase activity. As a result, hydrogen peroxide or other free radicals accumulate causing serious oxidative damage to various cells and tissues. In individuals with galactosemia, the enzymes needed for the further metabolism of galactose (galactose-1-phosphate uridyltransferase) are severely diminished or missing entirely, leading to toxic levels of galactose 1-phosphate, galactitol, and galactonate. High levels of galactitol in infants are specifically associated with hepatomegaly (an enlarged liver), cirrhosis, renal failure, cataracts, vomiting, seizure, hypoglycemia, lethargy, brain damage, and ovarian failure. Outside of the human body, Galactitol has been detected, but not quantified in, several different foods, such as common buckwheats, winter squash, calabash, black walnuts, and peanuts. This could make galactitol a potential biomarker for the consumption of these foods. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2R,3S,4R,5S)-Hexane-1,2,3,4,5,6-hexol | ChEBI | | D-Dulcitol | ChEBI | | D-Galactitol | ChEBI | | Dulcitol | ChEBI | | Dulcose | ChEBI | | Euonymit | ChEBI | | L-Galactitol | ChEBI | | Melampyrin | ChEBI | | Melampyrit | ChEBI | | Ambap5938 | HMDB | | Dulcite | HMDB | | Hexitol | HMDB | | Melampyrite | HMDB | | Melampyrum | HMDB | | meso-Galactitol | HMDB |
|
|---|
| Chemical Formula | C6H14O6 |
|---|
| Average Molecular Weight | 182.17 |
|---|
| Monoisotopic Molecular Weight | 182.079 |
|---|
| IUPAC Name | (2R,3S,4R,5S)-hexane-1,2,3,4,5,6-hexol |
|---|
| Traditional Name | galactitol |
|---|
| CAS Registry Number | 608-66-2 |
|---|
| SMILES | OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CO |
|---|
| InChI Identifier | InChI=1S/C6H14O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3-12H,1-2H2/t3-,4+,5+,6- |
|---|
| InChI Key | FBPFZTCFMRRESA-GUCUJZIJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as sugar alcohols. These are hydrogenated forms of carbohydrate in which the carbonyl group (aldehyde or ketone, reducing sugar) has been reduced to a primary or secondary hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sugar alcohol
- Monosaccharide
- Secondary alcohol
- Polyol
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Physiological effect | Health effect: |
|---|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Indirect biological role: Biological role: Industrial application: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 189.5 °C | Not Available | | Boiling Point | 275-280 °C | Wikipedia | | Water Solubility | 31 mg/mL at 15 °C | YALKOWSKY,SH & DANNENFELSER,RM (1992) | | logP | -3.10 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| GC-MS | Galactitol, non-derivatized, GC-MS Spectrum | splash10-014j-0941000000-61141f9f2ddc66e0b016 | Spectrum | | GC-MS | Galactitol, 6 TMS, GC-MS Spectrum | splash10-0gb9-1983000000-0a0dafcfea843fbb3c72 | Spectrum | | GC-MS | Galactitol, non-derivatized, GC-MS Spectrum | splash10-014j-0941000000-61141f9f2ddc66e0b016 | Spectrum | | GC-MS | Galactitol, non-derivatized, GC-MS Spectrum | splash10-0gb9-1983000000-0a0dafcfea843fbb3c72 | Spectrum | | Predicted GC-MS | Galactitol, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-03k9-9400000000-7a0f08ea65cbca1920f5 | Spectrum | | Predicted GC-MS | Galactitol, 6 TMS, Predicted GC-MS Spectrum - 70eV, Positive | splash10-0a6s-7141193000-dcd9245e12314ee0ced4 | Spectrum | | Predicted GC-MS | Galactitol, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_1_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_1_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_1_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_1_4, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_1_5, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_1_6, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_2_1, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_2_2, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_2_3, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_2_4, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_2_5, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_2_6, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_2_7, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_2_8, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_2_9, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_2_10, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Galactitol, TMS_2_11, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, N/A (Annotated) | splash10-005a-5900000000-35da0de23de5b9170aed | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, N/A (Annotated) | splash10-0a4i-9000000000-552b84764ceb9b497a7e | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, N/A (Annotated) | splash10-052f-9000000000-dfb4eef021d94d514588 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QqQ 4V, negative | splash10-001i-0900000000-028ff289dc870279e218 | 2020-07-21 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QqQ 8V, negative | splash10-001i-1900000000-a99ee988b2a1bbab9137 | 2020-07-21 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QqQ 12V, negative | splash10-0uyr-6900000000-04315425478b660c8947 | 2020-07-21 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QqQ 16V, negative | splash10-0kg9-9400000000-54350b0516f39f8c9b50 | 2020-07-21 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QqQ 20V, negative | splash10-0abi-9100000000-b4cf2b00830b765d5dcb | 2020-07-21 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - n/a 12V, negative | splash10-0089-9000000000-20463a683f8ab7aa9e4d | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - n/a 12V, negative | splash10-0a4i-9000000000-5c4795e954d5c3e9b74e | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - n/a 12V, negative | splash10-0udi-0900000000-43b7e571c1185de1e99b | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - n/a 12V, negative | splash10-00di-9000000000-160eaf7ef39d08853738 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - n/a 12V, negative | splash10-0udi-3900000000-3114e2bfe0cdbc68be19 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - n/a 12V, negative | splash10-000j-9000000000-635a869063bea88c0091 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - n/a 12V, negative | splash10-0uk9-3900000000-2ecc694b3a9844ec3ee5 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Orbitrap 3V, negative | splash10-03di-0900000000-61d17fd37e0e3ac16e37 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Orbitrap 4V, negative | splash10-03di-1900000000-90b308bca6baee5ab018 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Orbitrap 5V, negative | splash10-0ik9-2900000000-eb87481835668a3b0d3d | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Orbitrap 6V, negative | splash10-0w29-5900000000-a78bc11bf7d88c2b913e | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Orbitrap 7V, negative | splash10-0udi-8900000000-0ae9e05f3f4517635bba | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Orbitrap 9V, negative | splash10-0zmi-9500000000-4156ee30d358f68b2590 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - n/a 12V, negative | splash10-0udi-3900000000-0370fdfaee1df82b525d | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - n/a 12V, negative | splash10-05fr-9000000000-9699f16da2c5fbaef32d | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - n/a 12V, negative | splash10-000j-9000000000-0a119385f247c8ed7ae4 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, negative | splash10-014i-0490000000-403aabe62ebe0fa60ab3 | 2020-07-22 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 125 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, experimental) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Galactose Metabolism | 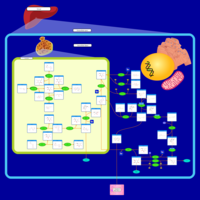 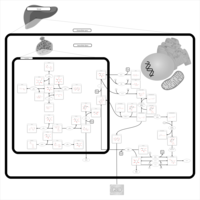 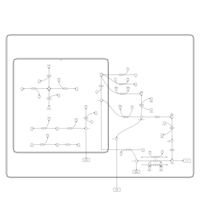 | 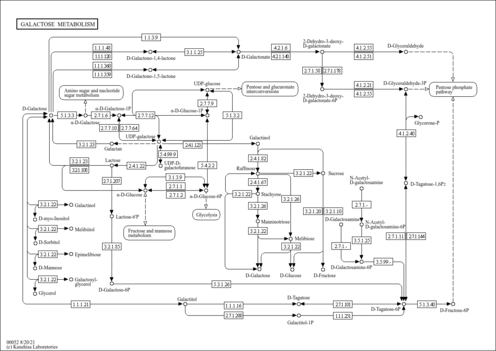 | | Galactosemia | 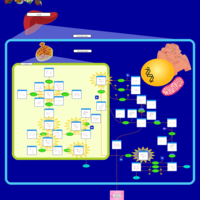 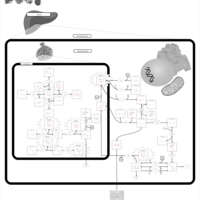 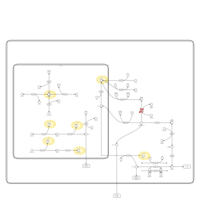 | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
|---|
| Transporters | Not Available |
|---|
| Metal Bindings | Not Available |
|---|
| Receptors | |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0000107 |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB006453 |
|---|
| KNApSAcK ID | C00001160 |
|---|
| Chemspider ID | 11357 |
|---|
| KEGG Compound ID | C01697 |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | 38183 |
|---|
| Wikipedia Link | Galactitol |
|---|
| METLIN ID | 5148 |
|---|
| PubChem Compound | 11850 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 16813 |
|---|
| References |
|---|
| General References | Not Available |
|---|
