| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-04-27 16:55:51 UTC |
|---|
| Updated at | 2021-01-04 18:49:16 UTC |
|---|
| CannabisDB ID | CDB005780 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | Hexacosanoic acid |
|---|
| Description | Hexacosanoic acid, also known as C26:0 or Cerotic acid, is a 26-carbon, long-chain saturated fatty acid. It belongs to the class of organic compounds known as very long-chain fatty acids. These are fatty acids with an aliphatic tail that contains at least 22 carbon atoms. Hexacosanoic acid is a very hydrophobic molecule that is practically insoluble in water. It exists as a white or beige crystalline solid and is most commonly found in beeswax and carnauba wax. Hexacosanoic acid has also been detected, but not quantified, in peanuts. Hexacosanoic acid exists in all eukaryotes, ranging from yeast to plants to humans. In humans, hexacosanoic acid is involved in beta oxidation of very long chain fatty acids. Hexacosanoic acid, with regard to humans, has been found to be associated with several diseases such as peroxisomal disorders, adrenomyeloneuropathy, and mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma. Hexacosanoic acid has also been linked to several inborn metabolic disorders including adrenoleukodystrophy (ALD) and peroxisomal biogenesis defect. ALD is characterized by normal development in early childhood, followed by rapid degeneration to a vegetative state. For the childhood cerebral form, stem cell transplant and gene therapy are options if the disease is detected early in the clinical course. Clinically, ALD is a heterogenous disorder, presenting with several distinct phenotypes, and no clear pattern of genotype-phenotype correlation. Treatment options for adrenoleukodystrophy (ALD) are limited. Adrenal insufficiency in ALD patients can be successfully treated. Approximately two-thirds of ALD patients will present with the childhood cerebral form of the disease, which is the most severe form. ALD is a peroxisomal disorder biochemically characterized by the accumulation of very long chain fatty acids (VLCFA), particularly hexacosanoic acid (C(26:0)) and tetracosanoic acid (C(24:0)), in tissues and biological fluids. The exact mechanism of the pathogenesis of the various forms of ALD is not known. Hexacosanoic acid is one of several acidic compounds found in cannabis smoke and is formed during the combustion of cannabis ( Ref:DOI ). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| C26:0 | ChEBI | | Ceratinic acid | ChEBI | | Ceric acid | ChEBI | | Cerinic acid | ChEBI | | Cerotic acid | ChEBI | | Cerotinic acid | ChEBI | | Cerylic acid | ChEBI | | CH3-[CH2]24-COOH | ChEBI | | Hexacosansaeure | ChEBI | | Hexacosoic acid | ChEBI | | Hexaeicosanoic acid | ChEBI | | N-C26:0 | ChEBI | | N-Hexacosanoic acid | ChEBI | | Ceratinate | Generator | | Cerate | Generator | | Cerinate | Generator | | Cerotate | Generator | | Cerotinate | Generator | | Cerylate | Generator | | Hexacosoate | Generator | | Hexaeicosanoate | Generator | | N-Hexacosanoate | Generator | | Hexacosanoate | Generator | | Hexacosanoate (N-C26:0) | HMDB | | Hexacosanoic acid | PhytoBank | | FA(26:0) | PhytoBank |
|
|---|
| Chemical Formula | C26H52O2 |
|---|
| Average Molecular Weight | 396.69 |
|---|
| Monoisotopic Molecular Weight | 396.3967 |
|---|
| IUPAC Name | hexacosanoic acid |
|---|
| Traditional Name | hexacosanoic acid |
|---|
| CAS Registry Number | 506-46-7 |
|---|
| SMILES | CCCCCCCCCCCCCCCCCCCCCCCCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C26H52O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23-24-25-26(27)28/h2-25H2,1H3,(H,27,28) |
|---|
| InChI Key | XMHIUKTWLZUKEX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as very long-chain fatty acids. These are fatty acids with an aliphatic tail that contains at least 22 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Very long-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Very long-chain fatty acid
- Straight chain fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Physiological effect | Health effect: |
|---|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Industrial application: Biological role: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 88.5 °C | Not Available | | Boiling Point | 250 °C | Wikipedia | | Water Solubility | Not Available | Not Available | | logP | Not Available | Not Available |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| EI-MS | Mass Spectrum (Electron Ionization) | splash10-0597-9201000000-41113a5b904c5db7332d | 2014-09-20 | View Spectrum | | GC-MS | Hexacosanoic acid, 1 TMS, GC-MS Spectrum | splash10-0159-2900100000-dc0324482e5853e76290 | Spectrum | | GC-MS | Hexacosanoic acid, non-derivatized, GC-MS Spectrum | splash10-0159-2900100000-dc0324482e5853e76290 | Spectrum | | GC-MS | Hexacosanoic acid, non-derivatized, GC-MS Spectrum | splash10-0159-2900100000-dc0324482e5853e76290 | Spectrum | | GC-MS | Hexacosanoic acid, non-derivatized, GC-MS Spectrum | splash10-0159-1900000000-9d83fa08f63878dd9794 | Spectrum | | Predicted GC-MS | Hexacosanoic acid, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-0006-8890000000-a358b076f9e4d3b08976 | Spectrum | | Predicted GC-MS | Hexacosanoic acid, 1 TMS, Predicted GC-MS Spectrum - 70eV, Positive | splash10-0fmr-9550000000-f90d4cc6a83ff8e16245 | Spectrum | | Predicted GC-MS | Hexacosanoic acid, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0a4j-0109000000-bc2c74027165503fd92f | 2012-07-25 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0pb9-2193000000-cf0ac4a96f99022c7b3a | 2012-07-25 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0w30-6096000000-65025824de97c5fb5ff7 | 2012-07-25 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - n/a 27V, negative | splash10-00lr-0009000000-998e2947790abd467bc7 | 2020-07-21 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0009000000-bd3c909a0431684b20e7 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ug1-3439000000-ca5336c838aa14069626 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-6974000000-93ebd8f603ed3f48dd9d | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-50e9c7751bdac41b78c8 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6t-0009000000-fdc9aa019c323235312e | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9113000000-af814ec2317a697df1a0 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-87ff11014d638fb7aedd | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-1009000000-28b98f74e9fa6d052c38 | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9003000000-9b4d6e9900769e9411a5 | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-2009000000-459e9bac675c0081de3d | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056s-7039000000-16142145274003e6395d | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9010000000-33f6f730f4cbdee5a975 | 2021-09-22 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, CDCl3, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 90 MHz, CDCl3, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CDCl3, experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Beta Oxidation of Very Long Chain Fatty Acids |    | 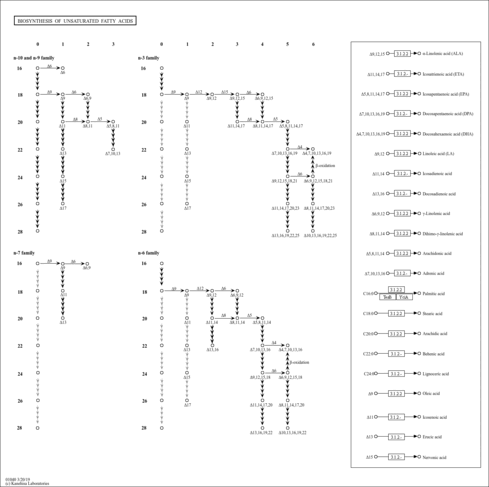 | | Adrenoleukodystrophy, X-linked |    | Not Available | | Carnitine-acylcarnitine translocase deficiency |    | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
|---|
| Transporters | Not Available |
|---|
| Metal Bindings | Not Available |
|---|
| Receptors | Not Available |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0002356 |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB004035 |
|---|
| KNApSAcK ID | C00035114 |
|---|
| Chemspider ID | 10037 |
|---|
| KEGG Compound ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | 1459812 |
|---|
| Wikipedia Link | Cerotic acid |
|---|
| METLIN ID | 6642 |
|---|
| PubChem Compound | 10469 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 31009 |
|---|
| References |
|---|
| General References | Not Available |
|---|
