| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-04-17 19:28:57 UTC |
|---|
| Updated at | 2020-12-07 19:11:54 UTC |
|---|
| CannabisDB ID | CDB005296 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | 7-Methylxanthine |
|---|
| Description | 7-Methylxanthine, also known as heteroxanthin, belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. 7-Methylxanthine is an extremely weak basic (essentially neutral) compound (based on its pKa). 7-Methylxanthine exists in all living organisms, ranging from bacteria to humans. Within humans, 7-methylxanthine participates in a number of enzymatic reactions. In particular, 7-methylxanthine and formaldehyde can be biosynthesized from paraxanthine through the action of the enzyme cytochrome P450 1A2. In addition, 7-methylxanthine can be converted into 7-methyluric acid through the action of the enzyme xanthine dehydrogenase/oxidase. In humans, 7-methylxanthine is involved in caffeine metabolism. Outside of the human body, 7-Methylxanthine has been detected, but not quantified in, arabica coffee. This could make 7-methylxanthine a potential biomarker for the consumption of these foods. 7-Methylxanthine is expected to be in Cannabis as all living plants are known to produce and metabolize it. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,7-Dihydro-7-methyl-1H-purine-2,6-dione | ChEBI | | 7-Methylxanthin | ChEBI | | Heteroxanthin | ChEBI | | Heteroxanthine | ChEBI | | 7-Methylxanthine, 7-(13)C-labeled | MeSH | | 2,6-Dihydroxy-7-methylpurine | HMDB | | 7-Methyl-3,7-dihydro-1H-purine-2,6-dione | HMDB | | 7-Methyl-7H-purine-2,6-diol | HMDB | | Methylxanthine | HMDB |
|
|---|
| Chemical Formula | C6H6N4O2 |
|---|
| Average Molecular Weight | 166.14 |
|---|
| Monoisotopic Molecular Weight | 166.0491 |
|---|
| IUPAC Name | 7-methyl-2,3,6,7-tetrahydro-1H-purine-2,6-dione |
|---|
| Traditional Name | 7-methylxanthine |
|---|
| CAS Registry Number | 552-62-5 |
|---|
| SMILES | CN1C=NC2=C1C(=O)NC(=O)N2 |
|---|
| InChI Identifier | InChI=1S/C6H6N4O2/c1-10-2-7-4-3(10)5(11)9-6(12)8-4/h2H,1H3,(H2,8,9,11,12) |
|---|
| InChI Key | PFWLFWPASULGAN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Xanthines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthine
- Alkaloid or derivatives
- Hydroxypyrimidine
- Pyrimidine
- N-substituted imidazole
- Heteroaromatic compound
- Imidazole
- Azole
- Azacycle
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Biological role: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | > 300 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | logP | -0.89 | GASPARI,F & BONATI,M (1987) |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | 7-Methylxanthine, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-00rb-4900000000-5a121544b907c24f5972 | Spectrum | | Predicted GC-MS | 7-Methylxanthine, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, N/A (Annotated) | splash10-014i-0900000000-30f6b1fe21e6924b4408 | 2012-07-25 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, N/A (Annotated) | splash10-00xr-6900000000-f5c1b1369f226330b60e | 2012-07-25 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, N/A (Annotated) | splash10-014i-9000000000-3b1edad37f3a300fd3b7 | 2012-07-25 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF 10V, positive | splash10-014i-0900000000-3c4effaf7fee8c2b61ea | 2020-07-21 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF 20V, positive | splash10-014i-0900000000-e2df10d0ab191cf48ad3 | 2020-07-21 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF 40V, positive | splash10-007k-9000000000-b0fa1ce6138138a94167 | 2020-07-21 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - n/a 11V, negative | splash10-0fk9-0900000000-45a47f7201f1b1cc4c0a | 2020-07-21 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QTOF 2V, positive | splash10-004i-3900000000-6b031051442287925863 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QTOF 4V, positive | splash10-0059-7900000000-8472e8c51cabdb800b2b | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QTOF 10V, positive | splash10-001i-9100000000-68f333821040ae2d706c | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QTOF 12V, positive | splash10-001i-9100000000-3c01ab92dda7a36ca993 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QTOF 14V, positive | splash10-001i-9100000000-92ec6b69d4cfe4cd2a3d | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QTOF 16V, positive | splash10-001i-9200000000-1a9b42f2cd8d64ed5d47 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QTOF 18V, positive | splash10-001i-9000000000-e26454dd03e8fe73c4a8 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QTOF 26V, positive | splash10-001i-9000000000-9e6003927514a8801358 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QTOF 30V, positive | splash10-001i-9000000000-fa3240e26cb87ab70fa4 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QTOF 35V, positive | splash10-001l-9000000000-9419618135a9b903f29c | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - QTOF 40V, positive | splash10-001l-9000000000-2aa69c9cec10c58e1b56 | 2020-07-22 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Orbitrap 3V, positive | splash10-014i-0900000000-ce6eec4ee9efe1c5a732 | 2020-07-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-d0d15cf114e854cb9389 | 2017-09-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01b9-1900000000-a22c299c1d6da4639631 | 2017-09-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kg-9200000000-5a65dfd385a5e35051c8 | 2017-09-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-1fd502b43c7af78c68ad | 2017-09-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9400000000-93978f7127dd2e954aa0 | 2017-09-01 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-212eeb1212b80abd05eb | 2017-09-01 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, 5%_DMSO, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 5%_DMSO, experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Caffeine Metabolism |    | 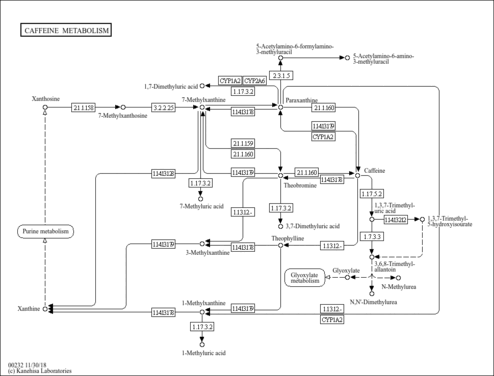 |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
|---|
| Transporters | Not Available |
|---|
| Metal Bindings | |
|---|
| Receptors | Not Available |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0001991 |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB001978 |
|---|
| KNApSAcK ID | C00007326 |
|---|
| Chemspider ID | 61660 |
|---|
| KEGG Compound ID | C16353 |
|---|
| BioCyc ID | 7-METHYLXANTHINE |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Xanthine |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 68374 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 48991 |
|---|
| References |
|---|
| General References | Not Available |
|---|
