| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-04-17 18:42:56 UTC |
|---|
| Updated at | 2020-12-07 19:11:06 UTC |
|---|
| CannabisDB ID | CDB004847 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | beta-Carotene |
|---|
| Description | beta-Carotene, also known as b-karotin or β-carotene, belongs to the class of organic compounds known as carotenes. These are a type of unsaturated hydrocarbons containing eight consecutive isoprene units. They are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Thus, beta-carotene is considered to be an isoprenoid lipid molecule. beta-Carotene is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. beta-Carotene is expected to be in Cannabis as all living plants are known to produce and metabolize it. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,1'-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-Tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl]bis(2,6,6-trimethylcyclohexene) | ChEBI | | all-trans-beta-Carotene | ChEBI | | beta-Karotin | ChEBI | | all-trans-b-Carotene | Generator | | all-trans-Β-carotene | Generator | | b-Karotin | Generator | | Β-karotin | Generator | | b-Carotene | Generator | | Β-carotene | Generator | | (all-e)-1,1'-(3,7,12,16-Tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis | HMDB | | (all-e)-1,1'-(3,7,12,16-Tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis[2,6,6-trimethyl-cyclohexene | HMDB | | all-e-b-Carotene | HMDB | | all-epsilon-beta-Carotene | HMDB | | Betacarotene | HMDB | | BetaVit | HMDB | | Carotaben | HMDB | | Carotene base 80S | HMDB | | FOOD Orange 5 | HMDB | | KPMK | HMDB | | Lucaratin | HMDB | | Lucarotin | HMDB | | Lurotin | HMDB | | Provatene | HMDB | | Provatenol | HMDB | | Rovimix b-carotene | HMDB | | Serlabo | HMDB | | Solatene | HMDB | | Hermal brand OF betacarotene | HMDB | | Max-caro | HMDB | | Merck brand OF betacarotene | HMDB | | Roche brand OF betacarotene | HMDB | | BellaCarotin | HMDB | | Betacarotene roche brand | HMDB | | Betacarotene solgar brand | HMDB | | Marlyn brand OF betacarotene | HMDB | | Betacarotene hermal brand | HMDB | | Max caro | HMDB | | Solgar brand OF betacarotene | HMDB | | 3m Brand OF betacarotene | HMDB | | Betacarotene 3m brand | HMDB | | Betacarotene marlyn brand | HMDB | | Betacarotene merck brand | HMDB | | Carotene, beta | HMDB | | MaxCaro | HMDB | | Vetoron | HMDB | | beta Carotene | HMDB | | b Carotene | HMDB | | Β carotene | HMDB | | beta-Carotene | HMDB |
|
|---|
| Chemical Formula | C40H56 |
|---|
| Average Molecular Weight | 536.89 |
|---|
| Monoisotopic Molecular Weight | 536.4382 |
|---|
| IUPAC Name | 1,3,3-trimethyl-2-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethylcyclohex-1-en-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl]cyclohex-1-ene |
|---|
| Traditional Name | β-carotene |
|---|
| CAS Registry Number | 7235-40-7 |
|---|
| SMILES | C\C(\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C=C(\C)/C=C/C=C(\C)/C=C/C1=C(C)CCCC1(C)C |
|---|
| InChI Identifier | InChI=1S/C40H56/c1-31(19-13-21-33(3)25-27-37-35(5)23-15-29-39(37,7)8)17-11-12-18-32(2)20-14-22-34(4)26-28-38-36(6)24-16-30-40(38,9)10/h11-14,17-22,25-28H,15-16,23-24,29-30H2,1-10H3/b12-11+,19-13+,20-14+,27-25+,28-26+,31-17+,32-18+,33-21+,34-22+ |
|---|
| InChI Key | OENHQHLEOONYIE-JLTXGRSLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as carotenes. These are a type of unsaturated hydrocarbons containing eight consecutive isoprene units. They are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Carotenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Carotene
- Branched unsaturated hydrocarbon
- Cycloalkene
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Industrial application: Biological role: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 183 °C | Not Available | | Boiling Point | 654.7 °C at 760 mmHg | Wikipedia | | Water Solubility | 0.6 mg/mL | Not Available | | logP | 14.764 | Wikipedia |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| EI-MS | Mass Spectrum (Electron Ionization) | splash10-05mx-9810010000-9a261fb8602890c2481e | 2015-03-01 | View Spectrum | | Predicted GC-MS | beta-Carotene, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-00di-3211290000-f968c686d39ae0f0fc50 | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0016-2794670000-c5bc2a4dbcd0f12cbf7f | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0002-2687970000-782d048d873c21fc6b82 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-05ai-1009510000-585581456b10671692bd | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - FAB-EBEB (JMS-HX/HX 110A, JEOL) , Positive | splash10-05mx-2920010000-471219f3e66bafb29bee | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - , positive | splash10-0006-0144900000-2fa7b609c7300ff8aba3 | 2017-09-14 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0434190000-0f2f0f2c4af76e6c3322 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000b-0679100000-6f4450c8ade1963f9201 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000j-1789100000-6f2a7024e42303e7737a | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000090000-cdea7c2d939f0c68b905 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0000090000-530c81799c9daabdc9bf | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0968170000-ec71ee19e546088e3b05 | 2016-09-12 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000j-0277960000-5b1eef16ffbc2ccd4b1e | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0081-0449400000-e362d306c4b151321fb6 | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0iki-0149200000-5b858e5700ff1d750a4e | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0001090000-b0a4df7c3417e82842d7 | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000j-0135090000-fb91b96b3605a056f12f | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0apj-0229000000-23e454829ef21fbedf66 | 2021-09-24 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CDCl3, experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Retinol Metabolism | 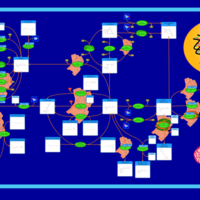 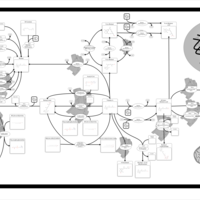 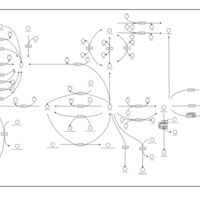 | 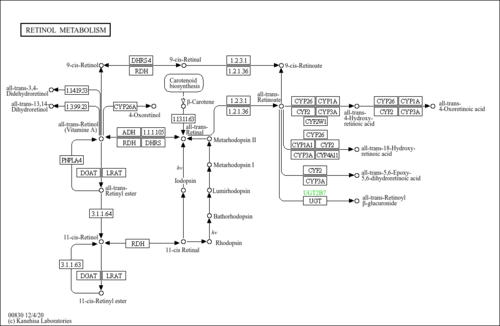 | | Vitamin A Deficiency | 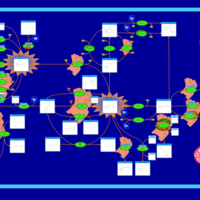 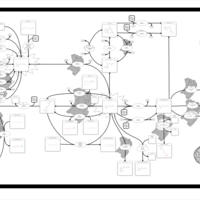 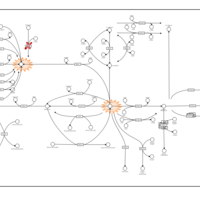 | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | |
|---|
| Transporters | Not Available |
|---|
| Metal Bindings | |
|---|
| Receptors | Not Available |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0000561 |
|---|
| DrugBank ID | DB06755 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB014613 |
|---|
| KNApSAcK ID | C00000919 |
|---|
| Chemspider ID | 4444129 |
|---|
| KEGG Compound ID | C02094 |
|---|
| BioCyc ID | CPD1F-129 |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Beta_Carotene |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 5280489 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 17579 |
|---|
| References |
|---|
| General References | Not Available |
|---|
