| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-03-30 20:39:12 UTC |
|---|
| Updated at | 2020-12-07 19:10:37 UTC |
|---|
| CannabisDB ID | CDB003358 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | LPA(22:0/0:0) |
|---|
| Description | LysoPA(22:0/0:0), also known as lpa(22:0), belongs to the class of organic compounds known as 1-acylglycerol-3-phosphates. These are lysophosphatidic acids where the glycerol is esterified with a fatty acid at O-1 position. LysoPA(22:0/0:0) is an extremely weak basic (essentially neutral) compound (based on its pKa). This compound is expected to be in Cannabis as all living plants are known to produce and metabolize it. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Behenoyl-glycero-3-phosphate | HMDB | | 1-Behenoyl-lysophosphatidic acid | HMDB | | LPA(22:0) | HMDB | | Lysophosphatidic acid(22:0/0:0) | HMDB | | Lysophosphatidic acid(22:0) | HMDB | | LPA(22:0/0:0) | HMDB | | 1-Docosanoyl-phosphatidic acid | HMDB | | 1-Docosanoyl-glycero-3-phosphate | HMDB | | 1-behenoyl-glycero-3-phosphate | SMPDB, HMDB | | 1-behenoyl-lysophosphatidic acid | SMPDB, HMDB | | LPA(22:0) | SMPDB, HMDB | | Lysophosphatidic acid(22:0/0:0) | SMPDB, HMDB |
|
|---|
| Chemical Formula | C25H51O7P |
|---|
| Average Molecular Weight | 494.65 |
|---|
| Monoisotopic Molecular Weight | 494.3372 |
|---|
| IUPAC Name | [3-(docosanoyloxy)-2-hydroxypropoxy]phosphonic acid |
|---|
| Traditional Name | 3-(docosanoyloxy)-2-hydroxypropoxyphosphonic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCCCCCCCCCCCCCCCCCCC(=O)OCC(O)COP(O)(O)=O |
|---|
| InChI Identifier | InChI=1S/C25H51O7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-25(27)31-22-24(26)23-32-33(28,29)30/h24,26H,2-23H2,1H3,(H2,28,29,30) |
|---|
| InChI Key | SQNPGTTUWWZACK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 1-acylglycerol-3-phosphates. These are lysophosphatidic acids where the glycerol is esterified with a fatty acid at O-1 position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphates |
|---|
| Direct Parent | 1-acylglycerol-3-phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-acylglycerol-3-phosphate
- Fatty acid ester
- Monoalkyl phosphate
- Fatty acyl
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Carboxylic acid ester
- Secondary alcohol
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
|
| Physiological effect | Organoleptic effect: |
|---|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Biological role: Industrial application: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | logP | Not Available | Not Available |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | LPA(22:0/0:0), 1 TMS, Predicted GC-MS Spectrum - 70eV, Positive | splash10-01ot-9430000000-a33ff2febbe599d5de27 | Spectrum | | Predicted GC-MS | LPA(22:0/0:0), non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | LPA(22:0/0:0), non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available | 2020-06-30 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000900000-5993ee9b6c84fd2abf4b | 2021-09-23 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0000900000-5993ee9b6c84fd2abf4b | 2021-09-23 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udu-2905600000-bc6c2183269b641ffde4 | 2021-09-23 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000900000-393b13e713960b451cd0 | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0000900000-393b13e713960b451cd0 | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-0905700000-d290b5722e3e6dbeaede | 2021-09-24 | View Spectrum |
|
|---|
| NMR | Not Available |
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | De Novo Triacylglycerol Biosynthesis TG(22:0/14:0/22:0) | 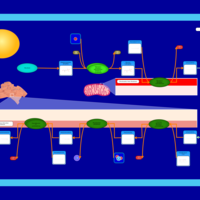 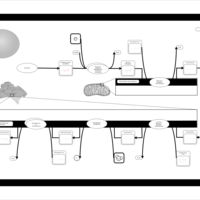 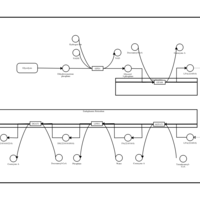 | Not Available | | De Novo Triacylglycerol Biosynthesis TG(22:0/14:0/24:0) | 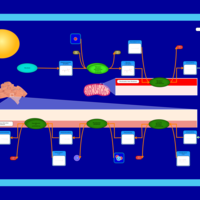 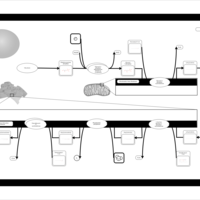 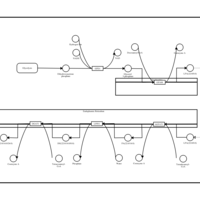 | Not Available | | De Novo Triacylglycerol Biosynthesis TG(22:0/14:0/14:1(9Z)) | 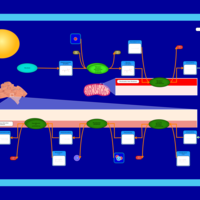 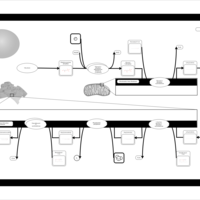 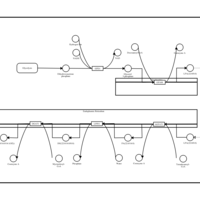 | Not Available | | De Novo Triacylglycerol Biosynthesis TG(22:0/14:0/16:1(9Z)) | 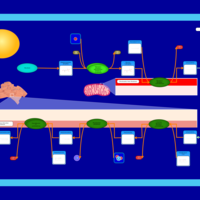 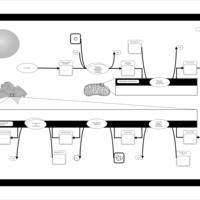 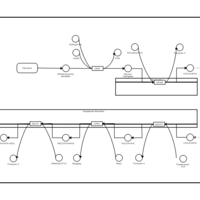 | Not Available | | De Novo Triacylglycerol Biosynthesis TG(22:0/14:0/18:1(11Z)) | 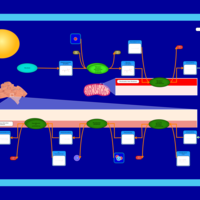 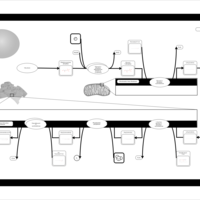 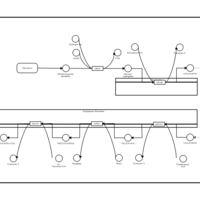 | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | Not Available |
|---|
| Transporters | Not Available |
|---|
| Metal Bindings | Not Available |
|---|
| Receptors | Not Available |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0114749 |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB095797 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 15921295 |
|---|
| KEGG Compound ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 17786745 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| References |
|---|
| General References | Not Available |
|---|