| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-03-19 00:51:46 UTC |
|---|
| Updated at | 2020-12-07 19:07:42 UTC |
|---|
| CannabisDB ID | CDB000698 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | 2-Propanone |
|---|
| Description | Acetone or 2-propanone, also known as dimethyl ketone, belongs to the class of organic compounds known as ketones. These are organic compounds in which a carbonyl group is bonded to two carbon atoms R2C=O (neither R may be a hydrogen atom). Ketones that have one or more alpha-hydrogen atoms undergo keto-enol tautomerization, the tautomer being an enol. Acetone is neutral molecule that is miscible in water. It readily dissolves in other organic solvents such as ethanol, methanol and diethyl ether. It occurs naturally in plants, trees, volcanic gases, forest fires, and is present in vehicle exhaust, alcoholic beverages, tobacco smoke, and landfill sites. Within the body small amounts of acetone are produced by the decarboxylation of ketone bodies (acetoacetate, beta-hydroxybutyrate). Certain dietary patterns, including prolonged fasting and high-fat low-carbohydrate dieting, can produce ketosis, in which acetone is formed in body tissue. More specifically, acetone can be produced from the oxidation of ingested isopropanol, or from the spontaneous/enzymatic breakdown of acetoacetate (a ketone body) in ketotic individuals. In humans acetone can be metabolized either by CYP2E1 via methylglyoxal to D-lactate and pyruvate (PMID 4285478 ). In addition to its presence in humans, acetone is found in a variety of foods and plants. Acetone has been rated as a generally recognized as safe (GRAS) substance when present in beverages, baked foods, desserts, and preserves at concentrations ranging from 5 to 8 mg/L. It is used as a flavor enhancer for sweetners in pickling mixes. Acetone is one of several ketones that are found in Cannabis sativa (PMID: 6991645 ). Acetone is widely used as an organic solvent and is the active ingredient in nail polish remover and as paint thinner. Acetone has been studied extensively and is believed to exhibit only slight toxicity in normal use. There is no strong evidence of chronic health effects if basic precautions are followed |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Propanone | ChEBI | | Aceton | ChEBI | | Azeton | ChEBI | | beta-Ketopropane | ChEBI | | Dimethyl ketone | ChEBI | | Dimethylcetone | ChEBI | | Dimethylketon | ChEBI | | Dimethylketone | ChEBI | | Methyl ketone | ChEBI | | Propanon | ChEBI | | Propanone | ChEBI | | Pyroacetic ether | ChEBI | | b-Ketopropane | Generator | | Β-ketopropane | Generator | | Dimethylformaldehyde | HMDB |
|
|---|
| Chemical Formula | C3H6O |
|---|
| Average Molecular Weight | 58.08 |
|---|
| Monoisotopic Molecular Weight | 58.0419 |
|---|
| IUPAC Name | propan-2-one |
|---|
| Traditional Name | acetone |
|---|
| CAS Registry Number | 67-64-1 |
|---|
| SMILES | CC(C)=O |
|---|
| InChI Identifier | InChI=1S/C3H6O/c1-3(2)4/h1-2H3 |
|---|
| InChI Key | CSCPPACGZOOCGX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as ketones. These are organic compounds in which a carbonyl group is bonded to two carbon atoms R2C=O (neither R may be a hydrogen atom). Ketones that have one or more alpha-hydrogen atoms undergo keto-enol tautomerization, the tautomer being an enol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Ketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ketone
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Physiological effect | Health effect: |
|---|
| Disposition | Route of exposure: Source: Biological location: |
|---|
| Role | Biological role: Environmental role: Industrial application: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | -94.8 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 1000 mg/mL at 25 °C | Not Available | | logP | -0.24 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| EI-MS | Mass Spectrum (Electron Ionization) | splash10-0006-9000000000-e24ae6a1f2f0e7aac3e7 | 2014-09-20 | View Spectrum | | GC-MS | Acetone, non-derivatized, GC-MS Spectrum | splash10-052f-9000000000-14f33068e4ba092deb77 | Spectrum | | GC-MS | Acetone, non-derivatized, GC-MS Spectrum | splash10-0006-9000000000-23fa1a3953b34b4d17db | Spectrum | | GC-MS | Acetone, non-derivatized, GC-MS Spectrum | splash10-0006-9000000000-5de48058c986d7c07ac3 | Spectrum | | GC-MS | Acetone, non-derivatized, GC-MS Spectrum | splash10-0002-9000000000-ac4e03d64a5d08cb0e2d | Spectrum | | GC-MS | Acetone, non-derivatized, GC-MS Spectrum | splash10-052f-9000000000-14f33068e4ba092deb77 | Spectrum | | GC-MS | Acetone, non-derivatized, GC-MS Spectrum | splash10-0006-9000000000-23fa1a3953b34b4d17db | Spectrum | | GC-MS | Acetone, non-derivatized, GC-MS Spectrum | splash10-0006-9000000000-5de48058c986d7c07ac3 | Spectrum | | GC-MS | Acetone, non-derivatized, GC-MS Spectrum | splash10-0002-9000000000-ac4e03d64a5d08cb0e2d | Spectrum | | Predicted GC-MS | Acetone, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | splash10-052f-9000000000-bc353e3a40f83275872b | Spectrum | | Predicted GC-MS | Acetone, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0a4i-9000000000-cbc26702df42f89389a4 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-052f-9000000000-76023af14a12c4b19cf9 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0a4l-9000000000-4a0900faf782fa344db9 | 2012-07-24 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - EI-B (JEOL JMS-D-3000) , Positive | splash10-052f-9000000000-cd6003561b339cd9243f | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80B) , Positive | splash10-0006-9000000000-23fa1a3953b34b4d17db | 2012-08-31 | View Spectrum | | MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-7M) , Positive | splash10-0006-9000000000-5de48058c986d7c07ac3 | 2012-08-31 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9000000000-31f47c962fee2ab51810 | 2015-04-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-29f0bd4f29c752b098b1 | 2015-04-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-44feb4a0693788e21f10 | 2015-04-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-9000000000-e3c53a345dc69a6403f2 | 2015-04-25 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-786aa0674415a8c32182 | 2015-04-25 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-3bc848ca563aa39df37e | 2015-04-25 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-9000000000-6e3379842f4da69e8e2b | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-6e3379842f4da69e8e2b | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-9251850c7854d63a048f | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9000000000-7d56b578e5d5ea65f901 | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9000000000-f1d310daeae27abc7672 | 2021-09-24 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-3f1c663ba1bd7d012172 | 2021-09-24 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 125 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, CDCl3, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Ketone Body Metabolism |    | 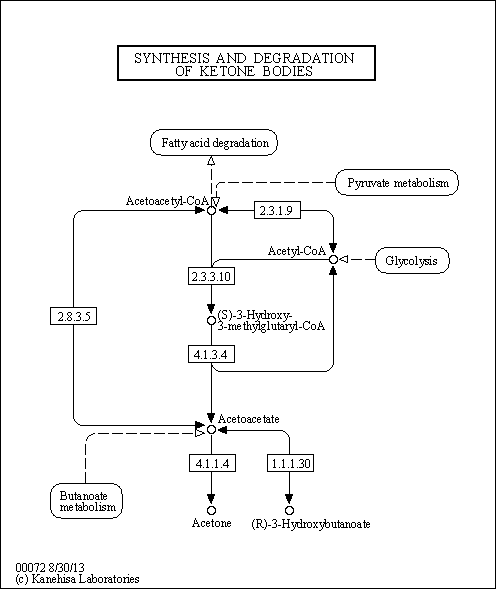 | | Succinyl CoA: 3-ketoacid CoA transferase deficiency |    | Not Available |
|
|---|
| Protein Targets |
|---|
| Enzymes | Not Available |
|---|
| Transporters | Not Available |
|---|
| Metal Bindings | Not Available |
|---|
| Receptors | Not Available |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0001659 |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB008301 |
|---|
| KNApSAcK ID | C00048304 |
|---|
| Chemspider ID | 175 |
|---|
| KEGG Compound ID | C00207 |
|---|
| BioCyc ID | ACETONE |
|---|
| BiGG ID | 34257 |
|---|
| Wikipedia Link | Acetone |
|---|
| METLIN ID | 3745 |
|---|
| PubChem Compound | 180 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 15347 |
|---|
| References |
|---|
| General References | - Turner CE, Elsohly MA, Boeren EG: Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod. 1980 Mar-Apr;43(2):169-234. doi: 10.1021/np50008a001. [PubMed:6991645 ]
- Miller ON, Bazzano G: Propanediol metabolism and its relation to lactic acid metabolism. Ann N Y Acad Sci. 1965 Jul 31;119(3):957-73. doi: 10.1111/j.1749-6632.1965.tb47455.x. [PubMed:4285478 ]
- Kalapos MP: Possible physiological roles of acetone metabolism in humans. Med Hypotheses. 1999 Sep;53(3):236-42. doi: 10.1054/mehy.1998.0752. [PubMed:10580530 ]
- Abou-Zeid AA, Fouad M, Yassein M: Microbiological production of acetone-butanol by Clostridium acetobutylicum. Zentralbl Bakteriol Naturwiss. 1978;133(2):125-34. doi: 10.1016/s0323-6056(78)80023-8. [PubMed:685531 ]
|
|---|
