| Record Information |
|---|
| Version | 1.0 |
|---|
| Created at | 2020-03-18 23:25:31 UTC |
|---|
| Updated at | 2022-12-13 23:36:25 UTC |
|---|
| CannabisDB ID | CDB000120 |
|---|
| Secondary Accession Numbers | Not Available |
|---|
| Cannabis Compound Identification |
|---|
| Common Name | Lysine |
|---|
| Description | Lysine and its bioactive form L-lysine, abbreviated Lys or L, is an essential amino acid. Normal requirements for adults are between 8 g per day or 12 mg/kg. Children and infants need more: 44 mg/kg per day for an eleven to-twelve-year old, and 97 mg/kg per day for three-to six-month old. Lysine is highly concentrated in muscle compared to most other amino acids. Normal lysine metabolism is dependent upon many nutrients including niacin, vitamin B6, riboflavin, vitamin C, glutamic acid and iron. Several inborn errors of lysine metabolism are known, such as cystinuria, hyperdibasic aminoaciduria I, lysinuric protein intolerance, propionic acidemia, and tyrosinemia I. Most are marked by mental retardation with occasional diverse symptoms such as absence of secondary sex characteristics, undescended testes, abnormal facial structure, anemia, obesity, enlarged liver and spleen, and eye muscle imbalance. Low lysine levels have been found in patients with Parkinson's, hypothyroidism, kidney disease, asthma and depression. The exact significance of these levels is unclear, yet lysine therapy can normalize these levels and has been associated with improvement of some patients with these conditions. Abnormally elevated hydroxylysines have been found in virtually all chronic degenerative diseases and coumadin therapy. The levels of this stress marker may be improved by high doses of vitamin C. Lysine is particularly useful in therapy for marasmus (wasting) and herpes simplex. It stops the growth of herpes simplex in culture and has helped to reduce the number and occurrence of cold sores in clinical studies. Beneficial clinical effects occurred with lysine doses ranging from 100 mg to 4 g a day. Higher doses may also be useful, and toxicity has not been reported in doses as high as 8 g per day. Diets high in lysine and low in arginine can be useful in the prevention and treatment of herpes as excess arginine antagonizes lysine. Lysine also may be a useful adjunct in the treatment of osteoporosis because it reduces calcium losses in urine. Although high protein diets result in loss of large amounts of calcium in urine, so does lysine deficiency. Lysine deficiency also may result in immunodeficiency. Requirements for this amino acid are probably increased by stress. Lysine is high in foods such as wheat germ, cottage cheese, chicken, wild game and pork. Less lysine is found in apple, apricot, bananas, avocados, guava, lime, brazil nuts, cashews, mung bean, fava bean and black bean as well as other seeds, nuts and fruits. Lysine is also found in cannabis plants (PMID: 6991645 ). Lysin is also a constituent of cannabis smok and is volatilized during the combustion of cannabis ( Ref:DOI ). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R)-2,6-Diaminohexanoic acid | ChEBI | | D-2,6-Diaminohexanoic acid | ChEBI | | D-Lysin | ChEBI | | DLY | ChEBI | | (R)-2,6-Diaminohexanoate | Generator | | D-2,6-Diaminohexanoate | Generator |
|
|---|
| Chemical Formula | C6H14N2O2 |
|---|
| Average Molecular Weight | 146.19 |
|---|
| Monoisotopic Molecular Weight | 146.1055 |
|---|
| IUPAC Name | (2R)-2,6-diaminohexanoic acid |
|---|
| Traditional Name | D-lysine |
|---|
| CAS Registry Number | 923-27-3 |
|---|
| SMILES | NCCCC[C@@H](N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H14N2O2/c7-4-2-1-3-5(8)6(9)10/h5H,1-4,7-8H2,(H,9,10)/t5-/m1/s1 |
|---|
| InChI Key | KDXKERNSBIXSRK-RXMQYKEDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as d-alpha-amino acids. These are alpha amino acids which have the D-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | D-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - D-alpha-amino acid
- Medium-chain fatty acid
- Amino fatty acid
- Fatty acid
- Fatty acyl
- Amino acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Organic nitrogen compound
- Amine
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
|
| Physiological effect | Health effect: |
|---|
| Disposition | Source: Biological location: |
|---|
| Role | Industrial application: |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 1.5 | Wikipedia | | logP | Not Available | Not Available |
|
|---|
| Predicted Properties | [] |
|---|
| Spectra |
|---|
| EI-MS/GC-MS | | Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Lysine, non-derivatized, Predicted GC-MS Spectrum - 70eV, Positive | Not Available | Spectrum |
|
|---|
| MS/MS | | Type | Description | Splash Key | View |
|---|
| Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f8a-2900000000-d5972dfce0860da55a0a | 2016-08-03 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-9700000000-d8e3259076e214ba9a3f | 2016-08-03 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a5c-9000000000-0b901a2eb0a82d41fc3d | 2016-08-03 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-603377bb44b83d433b77 | 2016-08-03 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-3900000000-e5d6ccb8cd7879780faf | 2016-08-03 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9100000000-e7f9a18ab48141069fee | 2016-08-03 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-94cae94fd691bdbb4d74 | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-600019f71b72fb289dee | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-bad9cb982e2df334ab55 | 2021-09-22 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-9600000000-dcc75a035223a66e7266 | 2021-09-23 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9000000000-b8581377af4d2519e68c | 2021-09-23 | View Spectrum | | Predicted MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-9e3d1f9dd58cb0efce55 | 2021-09-23 | View Spectrum |
|
|---|
| NMR | | Type | Description | | View |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | | Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | | Spectrum |
|
|---|
| Pathways |
|---|
| Pathways | | Name | SMPDB/Pathwhiz | KEGG | | Biotin Metabolism |    | 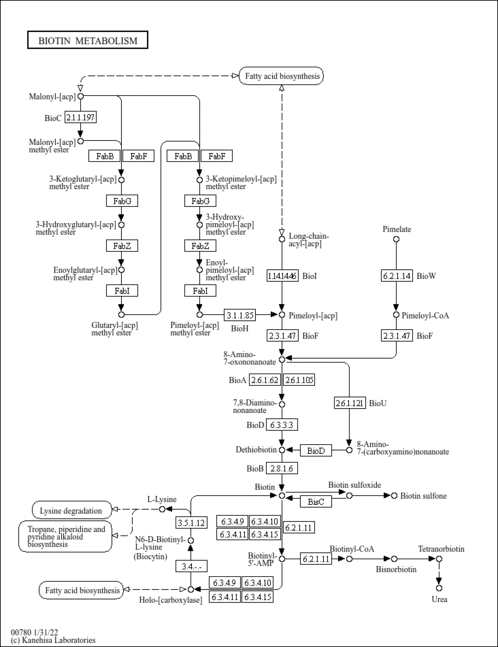 | | Biotinidase Deficiency |    | Not Available | | Multiple carboxylase deficiency, neonatal or early onset form |  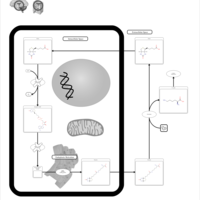  | Not Available | | Carnitine Synthesis | 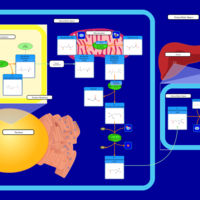 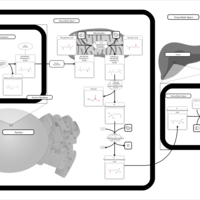 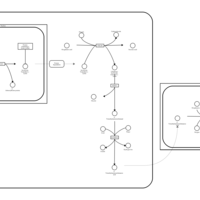 | Not Available | | Lysine Degradation |    | 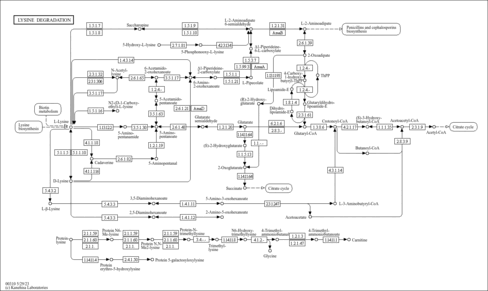 |
|
|---|
| Protein Targets |
|---|
| Enzymes | Not Available |
|---|
| Transporters | Not Available |
|---|
| Metal Bindings | Not Available |
|---|
| Receptors | Not Available |
|---|
| Transcriptional Factors | Not Available |
|---|
| Concentrations Data |
|---|
| |
| Alien Dawg | Detected and Quantified | 0.413 mg/g dry wt | | details | | Gabriola | Detected and Quantified | 0.246 mg/g dry wt | | details | | Island Honey | Detected and Quantified | 0.304 mg/g dry wt | | details | | Quadra | Detected and Quantified | 0.157 mg/g dry wt | | details | | Sensi Star | Detected and Quantified | 0.0993 mg/g dry wt | | details | | Tangerine Dream | Detected and Quantified | 0.203 mg/g dry wt | | details |
|
|---|
| External Links |
|---|
| HMDB ID | HMDB0003405 |
|---|
| DrugBank ID | DB03252 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FoodDB ID | FDB023163 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 51793 |
|---|
| KEGG Compound ID | C00739 |
|---|
| BioCyc ID | CPD-219 |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Lysine |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 57449 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 16855 |
|---|
| References |
|---|
| General References | - Turner CE, Elsohly MA, Boeren EG: Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod. 1980 Mar-Apr;43(2):169-234. doi: 10.1021/np50008a001. [PubMed:6991645 ]
|
|---|

